Abstract
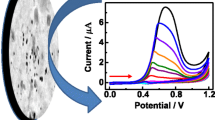
The electrocatalytic activity of silver nanoparticle-incorporated bentonite clay (Ag-Bt) for hydrogen peroxide (H2O2) reduction is investigated in 0.1 M pH 7.0 phosphate buffer solution. Ag-Bt material-coated glassy carbon (GC) electrode displays high electrocatalytic activity for H2O2 reduction with increased current response in comparison with GC/Bt electrode. The catalytic current increases linearly with incremental addition of H2O2 from 10 µM to 5.0 mM (based on the amperometric experiments at an applied potential −0.3 V). The apparent diffusion coefficient for H2O2 and catalytic rate constant for H2O2 reduction at the GC/Ag-Bt platform are calculated to be 2.3 × 10−5 cm2 s−1 and 2.20 × 104 M−1 s−1, respectively. The practical application using the Ag-Bt material is shown for the determination of H2O2 in real sample. The GC/Ag-Bt platform exhibits low detection limit (9.1 µM), high selectivity, reproducibility and stability.
Graphical abstract











Similar content being viewed by others
References
Safavi A, Maleki N, Farjamia E (2009) Electrodeposited silver nanoparticles on carbon ionic liquid electrode for electrocatalytic sensing of hydrogen peroxide. Electroanalysis 21:1533–1538
Mailloux RJ (2015) Teaching the fundamentals of electron transfer reactions in mitochondria and the production and detection of reactive oxygen species. Redox Biol 4:381–398
Song Y, Cui K, Wang L, Chen S (2009) The electrodeposition of Ag nanoparticles on a type I collagen modified glassy carbon electrode and their applications as a hydrogen peroxide sensor. Nanotechnology 20:105501–105508
Baga AN, Johnson GRA, Nazhat NB, Nazhat RAS (1988) A simple spectrophotometric determination of hydrogen peroxide at low concentrations in aqueous solution. Anal Chim Acta 204:349–353
Gimeno P, Bousquet C, Lassu N, Maggio AF, Civade C, Brenier C, Lempereur L (2015) High-performance liquid chromatography method for the determination of hydrogen peroxide present or released in teeth bleaching kits and hair cosmetic products. J Pharm Biomed Anal 107:386–393
Hurdis EC, Romeyn H (1954) Accuracy of determination of hydrogen peroxide by cerate oxidimetry. Anal Chem 26:320–325
Chairam S, Sroysee W, Boonchit C, Kaewprom C, Wangnoi TGN, Amatatongchai M, Jarujamrus P, Tamaung S, Somsook E (2015) Nonenzymatic sensor for hydrogen peroxide using a carbon paste electrode modified with a composite consisting of silver nanoparticles, poly(o-aminobenzoic acid) and magnetite. Int J Electrochem Sci 10:4611–4625
Sophia J, Muralidharan G (2014) Preparation of vinyl polymer stabilized silver nanospheres for electro-analytical determination of H2O2. Sens Actuators B 193:149–156
Hanaoka S, Lin JM, Yamada M (2001) Chemiluminescent flow sensor for H2O2 based on the decomposition of H2O2 catalyzed by cobalt(II)-ethanolamine complex immobilized on resin. Anal Chim Acta 426:57–64
Goesmann H, Feldmann C (2010) Nanoparticulate functional materials. Angew Chem Int Ed 49:1362–1395
Ramaraj R (2006) Nanostructured metal particle-modified electrodes for electrocatalytic and sensor applications. J Chem Sci 118:593–600
Zhang JJ, Liu YG, Jiang LP, Zhu JJ (2008) Synthesis, characterizations of silica-coated gold nanorods and its applications in electroanalysis of haemoglobin. Electrochem Commun 10:355–358
Praus P, Turicova M, Karlikova M, Kvitek L, Dvorsky R (2013) Nanocomposite of montmorillonite and silver nanoparticles: characterization and application in catalytic reduction of 4-nitrophenol. Mater Chem Phys 140:493–498
Rahman L, Shah A, Khan SB, Asiri AM, Hussain H, Han C, Qureshi R, Ashiq MN, Zia MA, Ishaq M, Kraatz HB (2015) Synthesis, characterization, and application of Au–Ag alloy nanoparticles for the sensing of an environmental toxin, pyrene. J Appl Electrochem 45:463–472
Rahman L, Shah A, Lunsford SK, Han C, Nadagouda MN, Demessie ES, Qureshi R, Khan MS, Kraatz HB, Dionysiou DD (2015) Monitoring of 2-butanone using a Ag–Cu bimetallic alloy nanoscale electrochemical sensor. RSC Adv 5:44427–44434
Rahman L, Qureshi R, Yasinzai MM, Shah A (2012) Synthesis and spectroscopic characterization of Ag–Cu alloy nanoparticles prepared in various ratios. Comptes Rendus Chim 15:533–538
Yang Y, Chen H, Zhao B, Bao X (2004) Size control of ZnO nanoparticles via thermal decomposition of zinc acetate coated on organic additives. J Cryst Growth 263:447–453
Guang-nian X, Xue-liang Q, Xiao-lin Q, Jian-guo C (2008) Preparation and characterization of stable monodisperse silver nanoparticles via photoreduction. Colloids Surf A 320:222–226
Khanna PK, Singh N, Charan S, Subbarao VVVS, Gokhale R, Mulik UP (2005) Synthesis and characterization of Ag/PVA nanocomposite by chemical reduction method. Mater Chem Phys 93:117–121
Rahman L, Shah A, Qureshi R, Khan SB, Asiri AM, Shah AHA, Ishaq M, Khan MS, Lunsford SK, Zia MA (2015) Spectroscopic analysis of Au–Cu alloy nanoparticles of various compositions synthesized by a chemical reduction method. Adv Mater Sci Eng 10:1155–1164
Aihara N, Torigoe K, Esumi K (1998) Preparation and characterization of gold and silver nanoparticles in layered laponite suspensions. Langmuir 14:4945–4949
Dong RX, Chou CC, Lin JJ (2009) Synthesis of immobilized silver nanoparticles on ionic silicate clay and observed low-temperature melting. J Mater Chem 19:2184–2188
Shah A, Rahman L, Qureshi R, Rehman Z (2012) Synthesis, characterization and applications of bimetallic (Au–Ag, Au–Pt, Au–Ru) alloy nanoparticles. Rev Adv Mater Sci 30:133–149
Mousty C (2004) Sensors and biosensors based on clay-modified electrodes-new trends. Appl Clay Sci 27:159–177
Tunc S, Duman O (2008) The effect of different molecular weight of poly(ethylene glycol) on the electrokinetic and rheological properties of Na-bentonite suspensions. Colloids Surf A 317:93–99
Mudrinic T, Mojovic Z, Nikolic AM, Bankovic P, Dojcinovic B, Vukelic N, Jovanovic D (2014) Beneficial effect of Ni in pillared bentonite based electrodes on the electrochemical oxidation of phenol. Electrochim Acta 144:92–99
Ganesan V, Ramaraj R (2000) In situ spectroelectrochemical studies of phenothiazine dyes at clay coated electrodes. J Electroanal Chem 490:54–61
Navratilova Z, Kula P (2003) Clay modified electrodes: present applications and prospects. Electroanalysis 15:837–846
Sonkar PK, Ganesan V (2015) Synthesis and characterization of silver nanoparticle-anchored amine-functionalized mesoporous silica for electrocatalytic determination of nitrite. J Solid State Electrochem 19:2107–2115
Azad UP, Prajapati N, Ganesan V (2015) Selective determination of isoniazid using bentonite clay modified electrodes. Bioelectrochemistry 101:120–125
Pal M, Ganesan V (2010) Electrochemical determination of nitrite using silver nanoparticles modified electrode. Analyst 135:2711–2716
Liu J, Lee JB, Kim DH, Kim Y (2007) Preparation of high concentration of silver colloidal nanoparticles in layered laponite sol. Colloids Surf A 302:276–279
Mallakpour S, Dinari M (2011) Preparation and characterization of new organoclays using natural amino acids and Cloisite Na+. Appl Clay Sci 51:353–359
Pol VG, Srivastava DN, Palchik O, Palchik V, Slifkin MA, Weiss AM, Gedanken A (2002) Sonochemical deposition of silver nanoparticles on silica spheres. Langmuir 18:3352–3357
Kumar PR, Viswanathan P, Ramaraj R (2014) Silicate sol–gel stabilized silver nanoparticles for sensor applications toward mercuric ions, hydrogen peroxide and nitrobenzene. Sens Actuators B 202:1070–1077
Azad UP, Turllapati S, Rastogi PK, Ganesan V (2014) Tris(1,10-phenanthroline)iron(II)-bentonite film as efficient electrochemical sensing platform for nitrite determination. Electrochim Acta 127:193–199
Heli H, Pishahang J (2014) Cobalt oxide nanoparticles anchored to multiwalled carbon nanotubes: synthesis and application for enhanced electrocatalytic reaction and highly sensitive nonenzymatic detection of hydrogen peroxide. Electrochim Acta 123:518–526
Hong HG, Mallouk TE (1991) Electrochemical measurements of electron transfer rates through zirconium 1,2-ethanediylbis(phosphonate) multilayer films on gold electrodes. Langmuir 7:2362–2369
Bard AJ, Faulkner LR (2006) Electrochemical methods fundamentals and applications. Wiley, India
Laviron E (1979) General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J Electroanal Chem 101:19–28
Selvaraju T, Sivagami S, Thangavel S, Ramaraj R (2008) Electrochemical and in situ spectroelectrochemical studies of gold nanoparticles immobilized Nafion matrix modified electrode. Bull Mater Sci 31:487–494
Heli H, Sattarahmady N, Vais RD, Mehdizadeh AR (2014) Enhanced electrocatalytic reduction and highly sensitive nonenzymatic detection of hydrogen peroxide using platinum hierarchical nanoflowers. Sens Actuators B 192:310–316
Xu B, Ye ML, Yu YX, Zhang WD (2010) A highly sensitive hydrogen peroxide amperometric sensor based on MnO2-modified vertically aligned multiwalled carbon nanotubes. Anal Chim Acta 674:20–26
Wang L, Zhu H, Song Y, Liu L, He Z, Wan L, Chen S, Xiang Y, Chen S, Chen J (2012) Architecture of poly(o-phenylenediamine)–Ag nanoparticle composites for a hydrogen peroxide sensor. Electrochim Acta 60:314–320
Bui MPN, Pham XH, Han KN, Li CA, Kim YS, Seong GH (2010) Electrocatalytic reduction of hydrogen peroxide by silver particles patterned on single-walled carbon nanotubes. Sens Actuators B 150:436–441
Han M, Liu S, Bao J, Dai Z (2012) Pd nanoparticle assemblies-As the substitute of HRP, in their biosensing applications for H2O2 and glucose. Biosens Bioelectron 31:151–156
Hrapovic S, Liu Y, Male KB, Luong JHT (2004) Electrochemical biosensing platforms using platinum nanoparticles and carbon nanotubes. Anal Chem 76:1083–1088
Liu Z, Zhao B, Shi Y, Guo C, Yang H, Li Z (2010) Novel nonenzymatic hydrogen peroxide sensor based on iron oxide–silver hybrid submicrospheres. Talanta 81:1650–1654
Lu W, Liao F, Luo Y, Chang G, Sun X (2011) Hydrothermal synthesis of well-stable silver nanoparticles and their application for enzymeless hydrogen peroxide detection. Electrochim Acta 56:2295–2298
Niu X, Zhao H, Chen C, Lan M (2012) Platinum nanoparticle-decorated carbon nanotube clusters on screen-printed gold nanofilm electrode for enhanced electrocatalytic reduction of hydrogen peroxide. Electrochim Acta 65:97–103
Su J, Gao F, Gu Z, Pien M, Sun H (2013) A novel 3-D fabrication of platinum nanoparticles decorated micro carbon pillars electrode for high sensitivity detection of hydrogen peroxide. Sens Actuators B 181:57–64
Acknowledgments
This study was financially supported by CSIR [01(2708)/13/EMR-II] and UGC [42-271/2013 (SR)], New Delhi. DKY acknowledges UGC for the junior research fellowship (JRF). We are thankful to Prof. C. R. Raj and Prof. S. A. John for useful suggestions and Prof. O. N. Srivastava, Banaras Hindu University for SEM and powder XRD facilities.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yadav, D.K., Gupta, R., Ganesan, V. et al. Electrochemical sensing platform for hydrogen peroxide determination at low reduction potential using silver nanoparticle-incorporated bentonite clay. J Appl Electrochem 46, 103–112 (2016). https://doi.org/10.1007/s10800-015-0904-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-015-0904-2