Abstract
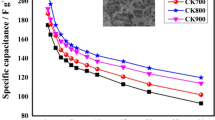
This paper presents the direct synthesis of highly porous carbons via the carbonization of potassium humate in inert atmosphere without any activation. Carbonization temperature is important for carbon structures and their electrochemical performance in electric double-layer capacitors. The CK800 material derived from potassium humate has a high–specific surface area of 890 m2 g−1 and an ideal average pore size of 2.56 nm. The CK800 material can also deliver a high-specific capacitance of 232 F g−1 (26.1 µF cm−2) at a constant charge/discharge current of 0.5 A g−1. This capacitance is maintained at a value of 114 F g−1 (12.8 µF cm−2) at 30 A g−1. This material also has good cycle stability with over 93.5 % capacitance retention after 5000 cycles when measured in a three-electrode system using 6 M KOH as electrolyte. In this study, the CK800 material is tested in a three-electrode system using 1 M NaHCO3 as electrolyte. Operational voltage in 1 M NaHCO3 aqueous solution could be extended up to 1.3 V.






Similar content being viewed by others
References
Morita M, Arizono R, Yoshimoto N, Egashira M (2014) On the electrochemical activation of alkali-treated soft carbon for advanced electrochemical capacitors. J Appl Electrochem 4:447–453
Zhang L, Jiang J, Holm N, Chen F (2014) Mini-chunk biochar supercapacitors. J Appl Electrochem 10:1145–1151. doi:10.1007/s10800-014-0726-7
Sun L, Wang C, Zhou Y, Zhang X, Qiu J (2014) KOH-activated depleted fullerene soot for electrochemical double-layer capacitors. J Appl Electrochem 2:309–316
Shukla A, Sampath S, Vijayamohanan K (2000) Electrochemical supercapacitors: energy storage beyond batteries. Curr Sci 12:1656–1661
Laskin J, Denisov EV, Shukla AK, Barlow SE, Futrell JH (2002) Surface-induced dissociation in a Fourier transform ion cyclotron resonance mass spectrometer: instrument design and evaluation. Anal Chem 14:3255–3261
Winter M, Brodd RJ (2004) What are batteries, fuel cells, and supercapacitors. Chem Rev 10:4245–4270
Burke A (2000) Ultracapacitors: why, how, and where is the technology. J Power Sources 1:37–50
Miller JR, Simon P (2008) Electrochemical capacitors for energy management. Sci Magazine 5889:651–652
Zhang LL, Zhao X (2009) Carbon-based materials as supercapacitor electrodes. Chem Soc Rev 9:2520–2531
Li L, Dong S, Chen X, Han P, Xu H, Yao J, Shang C, Liu Z, Cui G (2012) A renewable bamboo carbon/polyaniline composite for a high-performance supercapacitor electrode material. J Solid State Electrochem 3:877–882
Wei L, Sevilla M, Fuertes AB, Mokaya R, Yushin G (2011) Hydrothermal carbonization of abundant renewable natural organic chemicals for high-performance supercapacitor electrodes. Adv Energy Mater 3:356–361
Huang C, Grobert N, Watt AA, Johnston C, Crossley A, Young NP, Grant PS (2013) Layer-by-layer spray deposition and unzipping of single-wall carbon nanotube-based thin film electrodes for electrochemical capacitors. Carbon 61:525–536
Xu B, Zheng D, Jia M, Cao G, Yang Y (2013) Nitrogen-doped porous carbon simply prepared by pyrolyzing a nitrogen-containing organic salt for supercapacitors. Electrochim Acta 98:176–182. doi:10.1016/j.electacta.2013.03.053
Xu B, Duan H, Chu M, Cao G, Yang Y (2013) Facile synthesis of nitrogen-doped porous carbon for supercapacitors. J Mater Chem A 14:4565. doi:10.1039/c3ta01637d
Atkinson JD, Rood MJ (2012) Preparing microporous carbon from solid organic salt precursors using in situ templating and a fixed-bed reactor. Microporous Mesoporous Mater 160:174–181. doi:10.1016/j.micromeso.2012.05.008
Sevilla M, Fuertes AB (2013) A general and facile synthesis strategy towards highly porous carbons: carbonization of organic salts. J Mater Chem A 44:13738–13741
Sing KS, Gregg S (1982) Adsorption, surface area and porosity. Academic Press, London, pp 1–5
Stoeckli H, Rebstein P, Ballerini L (1990) On the assessment of microporosity in active carbons, a comparison of theoretical and experimental data. Carbon 28(6):907–909
He X, Zhang H, Zhang H, Li X, Xiao N, Qiu J (2014) Direct synthesis of 3D hollow porous graphene balls from coal tar pitch for high performance supercapacitors. J Mater Chem A 46:19633–19640
Zhang LL, Zhao X, Ji H, Stoller MD, Lai L, Murali S, Mcdonnell S, Cleveger B, Wallace RM, Ruoff RS (2012) Nitrogen doping of graphene and its effect on quantum capacitance, and a new insight on the enhanced capacitance of N-doped carbon. Energy Environ Science 11:9618–9625
Zhu Y, Murali S, Stoller MD, Ganesh K, Cai W, Ferreira PJ, Pirkle A, Wallace RM, Cychosz KA, Thommes M (2011) Carbon-based supercapacitors produced by activation of graphene. Science 6037:1537–1541
Kajdos A, Kvit A, Jones F, Jagiello J, Yushin G (2010) Tailoring the pore alignment for rapid ion transport in microporous carbons. J Am Cheml Soc 10:3252–3253
Biniak S, Szymański G, Siedlewski J, Światkowski A (1997) The characterization of activated carbons with oxygen and nitrogen surface groups. Carbon 12:1799–1810
Datsyuk V, Kalyva M, Papagelis K, Parthenios J, Tasis D, Siokou A, Kallitsis I, Galiotis C (2008) Chemical oxidation of multiwalled carbon nanotubes. Carbon 6:833–840
Okpalugo T, Papakonstantinou P, Murphy H, McLaughlin J, Brown N (2005) High resolution XPS characterization of chemical functionalised MWCNTs and SWCNTs. Carbon 1:153–161
Delpeux S, Beguin F, Benoit R, Erre R, Manolova N, Rashkov I (1998) Fullerene core star-like polymers—1. Preparation from fullerenes and monoazidopolyethers. Eur Polym J 7:905–915
Li W, Zhang F, Dou Y, Wu Z, Liu H, Qian X, Gu D, Xia Y, Tu B, Zhao D (2011) A self template strategy for the synthesis of mesoporous carbon nanofibers as advanced supercapacitor electrodes. Adv Energy Mater 3:382–386
Deng Y, Wei J, Sun Z, Zhao D (2013) Large-pore ordered mesoporous materials templated from non-Pluronic amphiphilic block copolymers. Chem Soc Rev 9:4054–4070
Chaikittisilp W, Hu M, Wang H, Huang H-S, Fujita T, Wu KC-W, Chen L-C, Yamauchi Y, Ariga K (2012) Nanoporous carbons through direct carbonization of a zeolitic imidazolate framework for supercapacitor electrodes. Chem Commun 58:7259–7261
Li F, Morris M, Chan K-Y (2011) Electrochemical capacitance and ionic transport in the mesoporous shell of a hierarchical porous core–shell carbon structure. J Mater Chem 24:8880–8886
Zhou G, Wang D-W, Li F, Zhang L, Li N, Wu Z-S, Wen L, Lu GQ, Cheng H-M (2010) Graphene-wrapped Fe3O4 anode material with improved reversible capacity and cyclic stability for lithium ion batteries. Chem Mater 18:5306–5313
Jiang HL, Liu B, Lan YQ, Kuratani K, Akita T, Shioyama H, Zong F, Xu Q (2011) From metal-organic framework to nanoporous carbon: toward a very high surface area and hydrogen uptake. J Am Chem Soc 31:11854–11857. doi:10.1021/ja203184k
Portet C, Yang Z, Korenblit Y, Gogotsi Y, Mokaya R, Yushin G (2009) Electrical double-layer capacitance of zeolite-templated carbon in organic electrolyte. J Electrochem Soc 1:A1. doi:10.1149/1.3002375
Wang Y, Chang B, Guan D, Dong X (2015) Mesoporous activated carbon spheres derived from resorcinol-formaldehyde resin with high performance for supercapacitors. J Solid State Electrochem 19(6):1783–1791
Kishore B, Shanmughasundaram D, Penki TR, Munichandraiah N (2014) Coconut kernel-derived activated carbon as electrode material for electrical double-layer capacitors. J Appl Electrochem 8:903–916
Luo H-M, Yang Y-F, Sun Y-X, Zhao X, Zhang J-Q (2015) Preparation of lactose-based attapulgite template carbon materials and their electrochemical performance. J Solid State Electrochem 19(4):1171–1180
Zhang P, Sun F, Shen Z, Cao D (2014) ZIF-derived porous carbon: a promising supercapacitor electrode material. J Mater Chem A 2(32):12873–12880
Stoller MD, Park S, Zhu Y, An J, Ruoff RS (2008) Graphene-based ultracapacitors. Nano Lett 10:3498–3502
Zhang J, Zhao X (2012) On the configuration of supercapacitors for maximizing electrochemical performance. ChemSusChem 5:818–841
Hulicova D, Kodama M, Hatori H (2006) Electrochemical performance of nitrogen-enriched carbons in aqueous and non-aqueous supercapacitors. Chem Mater 9:2318–2326
Demarconnay L, Raymundo-Pinero E, Béguin F (2010) A symmetric carbon/carbon supercapacitor operating at 1.6 V by using a neutral aqueous solution. Electrochem Commun 10:1275–1278
Fic K, Lota G, Meller M, Frackowiak E (2012) Novel insight into neutral medium as electrolyte for high-voltage supercapacitors. Energy Environ Sci 2:5842–5850
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (NSFC, Nos. 21364004 and 51462020).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luo, H., Yang, Y., Chen, Y. et al. Structure and electrochemical performance of highly porous carbons by single-step potassium humate carbonization for application in supercapacitors. J Appl Electrochem 46, 113–121 (2016). https://doi.org/10.1007/s10800-015-0894-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-015-0894-0