Abstract
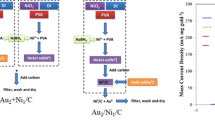
This study investigated the simultaneous in situ formation of Ni-based catalysts at the anode and cathode for glycerol oxidation and hydrogen evolution, respectively. The formation of electrocatalysts and their immobilization on the electrode surface occur simultaneously, avoiding the tedious and laborious procedures for the preparation and immobilization of the electrocatalysts. Ni salt in the homogeneous solution was deposited to repair the Ni-based electrocatalysts on the electrode surface, accompanied by the electrolysis of glycerol at the anode and the hydrogen evolution reaction at the cathode, exhibiting good working stability. This technique may find potential applications in the conversion of solar energy into storable fuels via the electrolysis of H2O or small molecules to produce hydrogen.





Similar content being viewed by others
References
Dau H, Limberg C, Reier T, Risch M, Roggan S, Strasser P (2010) The mechanism of water oxidation: from electrolysis via homogeneous to biological catalysis. ChemCatChem 2:724–761. doi:10.1002/cctc.201000126
Symes MD, Cronin L (2013) Decoupling hydrogen and oxygen evolution during electrolytic water splitting using an electron-coupled-proton buffer. Nat Chem 5:403–409. doi:10.1038/nchem.1621
Birry L, Lasia A (2004) Studies of the hydrogen evolution reaction on Raney nickel—molybdenum electrodes. J Appl Electrochem 34:735–749. doi:10.1023/B:JACH.0000031161.26544.6a
Marinović V, Stevanović J, Jugović B, Maksimović M (2006) Hydrogen evolution on Ni/WC composite coatings. J Appl Electrochem 36:1005–1009. doi:10.1007/s10800-006-9168-1
Le C (2011) A review of non-fossil energy based hydrogen production technologies. Energy Res Inform 27:130–137
Zhong C, Hu WB, Cheng YF (2013) Recent advances in electrocatalysts for electro-oxidation of ammonia. J Mater Chem A 1:3216–3238. doi:10.1039/C2TA00607C
Ding R, Qi L, Jia M, Wang H (2014) Facile synthesis of mesoporous spinel NiCo2O4 nanostructures as highly efficient electrocatalysts for urea electro-oxidation. Nanoscale 6:1369–1376. doi:10.1039/C3NR05359H
Li S-L, Xu Q (2013) Metal-organic frameworks as platforms for clean energy. Energy Environ Sci 6:1656–1683. doi:10.1039/C3EE40507A
Luo J, Njoki PN, Lin Y, Mott D, Wang Zhong C-J (2006) Characterization of carbon-supported AuPt nanoparticles for electrocatalytic methanol oxidation reaction. Langmuir 22:2892–2898. doi:10.1021/la0529557
Yang M, Cai Q, Liu C, Wu R, Sun D, Chen Y, Tang Y, Lu T (2014) Monodispersed hollow platinum nanospheres: facile synthesis and their enhanced electrocatalysis for methanol oxidation. J Mater Chem A 2:13738–13743. doi:10.1039/C4TA01434K
Liao H, Qiu Z, Wan Q, Wang Z, Liu Y, Yang N (2014) Universal electrode interface for electrocatalytic oxidation of liquid fuels. ACS Appl Mater Interfaces 6:18055–18062. doi:10.1021/am504926r
Zhou C-H, Beltramini JN, Fan Y-X, Lu GQ (2008) Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem Soc Rev 37:527–549. doi:10.1039/B707343G
Du P, Eisenberg R (2012) Catalysts made of earth-abundant elements (Co, Ni, Fe) for water splitting: recent progress and future challenges. Energy Environ Sci 5:6012–6021. doi:10.1039/C2EE03250C
Doyle RL, Godwin IJ, Brandon MP, Lyons MEG (2013) Redox and electrochemical water splitting catalytic properties of hydrated metal oxide modified electrodes. PCCP 15:13737–13783. doi:10.1039/C3CP51213D
Kanan MW, Surendranath Y, Nocera DG (2009) Cobalt-phosphate oxygen-evolving compound. Chem Soc Rev 38:109–114. doi:10.1039/B802885K
Dey S, Mondal B, Dey A (2014) An acetate bound cobalt oxide catalyst for water oxidation: role of monovalent anions and cations in lowering overpotential. PCCP 16:12221–12227. doi:10.1039/C4CP01205D
Yin Q, Tan JM, Besson C, Geletii YV, Musaev DG, Kuznetsov AE, Luo Z, Hardcastle KI, Hill CL (2010) A fast soluble carbon-free molecular water oxidation catalyst based on abundant metals. Science 328:342–345. doi:10.1126/science.1185372
Lei H, Han A, Li F, Zhang M, Han Y, Du P, Lai W, Cao R (2014) Electrochemical, spectroscopic and theoretical studies of a simple bifunctional cobalt corrole catalyst for oxygen evolution and hydrogen production. PCCP 16:1883–1893. doi:10.1039/C3CP54361G
Helm ML, Stewart MP, Bullock RM, DuBois MR, DuBois DL (2011) A synthetic nickel electrocatalyst with a turnover frequency above 100,000 s − 1 for H2 production. Science 333:863–866
Popczun EJ, McKone JR, Read CG, Biacchi AJ, Wiltrout AM, Lewis NS, Schaak RE (2013) Nanostructured nickel phosphide as an electrocatalyst for the hydrogen evolution reaction. J Am Chem Soc 135:9267–9270
Raj IA, Vasu K (1990) Transition metal-based hydrogen electrodes in alkaline solution—electrocatalysis on nickel based binary alloy coatings. J Appl Electrochem 20:32–38
Lupi C, Dell’Era A, Pasquali M (2009) Nickel–cobalt electrodeposited alloys for hydrogen evolution in alkaline media. Int J Hydrog Energy 34:2101–2106
Tian J, Liu Q, Cheng N, Asiri AM, Sun X (2014) Self-supported Cu3P nanowire arrays as an integrated high-performance three-dimensional cathode for generating hydrogen from water. Angew Chem Int Ed 53:9577–9581. doi:10.1002/anie.201403842
Irshad A, Munichandraiah N (2014) An oxygen evolution Co-Ac catalyst—the synergistic effect of phosphate ions. PCCP 16:5412–5422. doi:10.1039/C3CP54860K
Kanan MW, Nocera DG (2008) In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. Science 321:1072–1075. doi:10.1126/science.1162018
Oliveira VL, Morais C, Servat K, Napporn TW, Tremiliosi-Filho G, Kokoh KB (2013) Glycerol oxidation on nickel based nanocatalysts in alkaline medium—identification of the reaction products. J Electroanal Chem 703:56–62. doi:10.1016/j.jelechem.2013.05.021
Marshall AT, Haverkamp RG (2008) Production of hydrogen by the electrochemical reforming of glycerol–water solutions in a PEM electrolysis cell. Int J Hydrog Energy 33:4649–4654. doi:10.1016/j.ijhydene.2008.05.029
Gómez X, Fernández C, Fierro J, Sánchez ME, Escapa A, Morán A (2011) Hydrogen production: two stage processes for waste degradation. Bioresour Technol 102:8621–8627. doi:10.1016/j.biortech.2011.03.055
Yang Z, Miao Y, Wang T, Liang X, Xiao M, Li W, Yang Y (2014) The self-adsorption of Ni ultrathin layer on glassy carbon surface and their electrocatalysis toward glucose. J Electrochem Soc 161:H375–H378. doi:10.1149/2.049406jes
Marken F, Paddon CA, Asogan D (2002) Direct cytochrome c electrochemistry at boron-doped diamond electrodes. Electrochem Commun 4:62–66
Fernández L, Carrero H (2005) Electrochemical evaluation of ferrocene carboxylic acids confined on surfactant–clay modified glassy carbon electrodes: oxidation of ascorbic acid and uric acid. Electrochim Acta 50:1233–1240
Jeffery DZ, Camara GA (2010) The formation of carbon dioxide during glycerol electrooxidation in alkaline media: first spectroscopic evidences. Electrochem Commun 12:1129–1132. doi:10.1016/j.elecom.2010.06.001
Wang D, Yan W, Vijapur SH, Botte GG (2013) Electrochemically reduced graphene oxide–nickel nanocomposites for urea electrolysis. Electrochim Acta 89:732–736. doi:10.1016/j.electacta.2012.11.046
Wu M-S, Ji R-Y, Zheng Y-R (2014) Nickel hydroxide electrode with a monolayer of nanocup arrays as an effective electrocatalyst for enhanced electrolysis of urea. Electrochim Acta 144:194–199. doi:10.1016/j.electacta.2014.08.098
Miao Y, Ouyang L, Zhou S, Xu L, Yang Z, Xiao M, Ouyang R (2014) Electrocatalysis and electroanalysis of nickel, its oxides, hydroxides and oxyhydroxides toward small molecules. Biosens Bioelectron 53:428–439. doi:10.1016/j.bios.2013.10.008
Miao Y, Wu J, Zhou S, Yang Z, Ouyang R (2013) Synergistic effect of bimetallic Ag and Ni alloys on each other’s electrocatalysis to glucose oxidation. J Electrochem Soc 160:B47–B53. doi:10.1149/2.059304jes
Walczak MM, Popenoe DD, Deinhammer RS, Lamp BD, Chung C, Porter MD (1991) Reductive desorption of alkanethiolate monolayers at gold: a measure of surface coverage. Langmuir 7:2687–2693
Acknowledgments
The authors greatly appreciate the support from the Innovation Program of the Shanghai Municipal Education Commission (14ZZ139).
Author information
Authors and Affiliations
Corresponding author
Additional information
Xiaocai Liang, Mingshu Xiao and Minglu Xu contributed equally to this work and should be considered co-first authors.
Rights and permissions
About this article
Cite this article
Liang, X., Xiao, M., Xu, M. et al. Simultaneous in situ formation of Ni-based catalysts at the anode for glycerol oxidation and at the cathode for hydrogen evolution. J Appl Electrochem 46, 1–8 (2016). https://doi.org/10.1007/s10800-015-0888-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-015-0888-y