Abstract
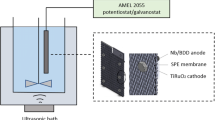
Methyl paraben is commonly employed as a preservative in pharmaceutical preparations, personal care products and some processed foods. However, the ester constitutes a potential pollutant in aquatic environments and has been classified as an endocrine disruptor. This study describes the degradation of methyl paraben (100 mg L−1 in 0.05 mol L−1 aqueous potassium sulfate at pH 5.7) by means of an electrochemical process (employing a boron-doped diamond anode) either alone or coupled with sonolysis. Electrolyses were performed at 25, 30 and 35 ± 1 °C during 120 min using applied constant current densities of 10.8 and 21.6 mA cm−2. The hybrid sonoelectrochemical processes were conducted under similar conditions with the application of ultrasound at a frequency of 20 kHz and a power intensity of 523 W cm−2. Although mineralization of methyl paraben could be achieved using either process, in comparison with the electrochemical method, the hybrid technique showed a higher mineralization efficiency (around 60 %) with approximately 50 % removal of total organic carbon, thereby confirming the synergistic effect of sonolysis.





Similar content being viewed by others
References
Liao C, Kannan K (2014) Concentrations and composition profiles of parabens in currency bills and paper products including sanitary wipes. Sci Total Environ 475:8–15
Andersen FA (2008) Final amended report on the safety assessment of methylparaben, ethylparaben, propylparaben, isopropylparaben, butylparaben, isobutylparaben, and benzylparaben as used in cosmetic products. Int J Toxicol 27(Suppl 4):1–82
Eriksson E, Andersen HR, Ledin A (2008) Substance flow analysis of parabens in Denmark complemented with a survey of presence and frequency in various commodities. J Hazard Mater 156:240–259
Soni MG, Carabin IG, Burdock GA (2005) Safety assessment of esters of p-hydroxybenzoic acid (parabens). Food Chem Toxicol 43:985–1015
Darbre PD, Aljarrah A, Miller WR, Coldham NG, Sauer MJ, Pope GS (2004) Concentrations of parabens in human breast tumours. J Appl Toxicol 24:5–13
Soni MG, Burdock GA, Taylor SL, Greenberg NA (2001) Safety assessment of propyl paraben: a review of the published literature. Food Chem Toxicol 39:513–532
Liao C, Lee S, Moon H-B, Yamashita N, Kannan K (2013) Parabens in sediment and sewage sludge from the United States, Japan, and Korea: spatial distribution and temporal trends. Environ Sci Technol 47:10895–10902
Liao C, Chen L, Kannan K (2013) Occurrence of parabens in foodstuffs from China and its implications for human dietary exposure. Environ Int 57–58:68–74
Dickerson SM, Gore AC (2007) Estrogenic environmental endocrine-disrupting chemical effects on reproductive neuroendocrine function and dysfunction across the life cycle. Rev Endocr Metab Disord 8:143–159
Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC (2009) Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev 30:293–342
Pugazhendhi D, Pope GS, Darbre PD (2005) Oestrogenic activity of p-hydroxybenzoic acid (common metabolite of paraben esters) and methylparaben in human breast cancer cell lines. J Appl Toxicol 25:301–309
Byford JR, Shaw LE, Drew MG, Pope GS, Sauer MJ, Darbre PD (2002) Oestrogenic activity of parabens in MCF7 human breast cancer cells. J Steroid Biochem Mol Biol 80:49–60
Lee HB, Peart TE, Svoboda ML (2005) Determination of endocrine-disrupting phenols, acidic pharmaceuticals, and personal-care products in sewage by solid-phase extraction and gas chromatography-mass spectrometry. J Chromatogr A 1094:122–129
Witorsch RJ, Thomas JA (2010) Personal care products and endocrine disruption: a critical review of the literature. Crit Rev Toxicol 40(Suppl 3):1–30
Kasprzyk-Hordern B, Dinsdale RM, Guwy AJ (2008) The occurrence of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs in surface water in South Wales, UK. Water Res 42:3498–3518
Githinji LJM, Musey MK, Ankumah RO (2011) Evaluation of the fate of ciprofloxacin and amoxicillin in domestic wastewater. Water Air Soil Pollut 219:191–201
Trovó AG, Nogueira RFP, Aguera A, Fernandez-Alba AR, Sirtori C, Malato S (2009) Degradation of sulfamethoxazole in water by solar photo-Fenton. Chemical and toxicological evaluation. Water Res 43:3922–3931
Stackelberg PE, Gibs J, Furlong ET, Meyer MT, Zaugg SD, Lippincott RL (2007) Efficiency of conventional drinking-water-treatment processes in removal of pharmaceuticals and other organic compounds. Sci Total Environ 377:255–272
Yin X, Xin F, Zhang F, Wang S, Zhang G (2006) Kinetic study on photocatalytic degradation of 4BS azo dye over TiO2 in slurry. Environ Eng Sci 23:1000–1008
Chacón JM, Leal MT, Sánchez M, Bandala ER (2006) Solar photocatalytic degradation of azo-dyes by photo-Fenton process. Dyes Pigments 69:144–150
Porter JJ, Snider EH (1976) Long-term biodegradability of textile chemicals. J Water Pollut Control Fed 48:2198–2210
Zhang G, Wang S, Zhao S, Fu L, Chen G, Yang F (2011) Oxidative degradation of azo dye by hydrogen peroxide electrogenerated in situ on anthraquinonemonosulphonate/polypyrrole composite cathode with heterogeneous CuO/gamma-Al2O3 catalyst. Appl Catal B Environ 106:370–378
Gupta VK, Jain R, Mittal A, Saleh TA, Nayak A, Agarwal S, Sikarwar S (2012) Photo-catalytic degradation of toxic dye amaranth on TiO2/UV in aqueous suspensions. Mater Sci Eng C 32:12–17
Panizza M, Cerisola G (2009) Direct and mediated anodic oxidation of organic pollutants. Chem Rev 109:6541–6569
Wang JL, Xu LJ (2012) Advanced oxidation processes for wastewater treatment: formation of hydroxyl radical and application. Crit Rev Environ Sci Technol 42:251–325
Brillas E, Sirés I, Oturan MA (2009) Electro-Fenton process and related electrochemical technologies based on Fenton’s reaction chemistry. Chem Rev 109:6570–6631
Steter JR, Rocha RS, Dionísio D, Lanza MRV, Motheo AJ (2014) Electrochemical oxidation route of methyl paraben on a boron-doped diamond anode. Electrochim Acta 117:127–133
Steter JR, Dionísio D, Miwa DW, Lanza MRV, Motheo AJ (2012) Electrochemical degradation of methyl paraben using a boron-doped diamond anode. ECS Trans 43:111–117
Barros WRP, Franco PC, Steter JR, Rocha RS, Lanza MRV (2014) Electro-Fenton degradation of the food dye amaranth using a gas diffusion electrode modified with cobalt (II) phthalocyanine. J Electroanal Chem 722–723:46–53
Reis RM, Baio JAF, Migliorini FL, Rocha RS, Baldan MR, Ferreira NG, Lanza MRV (2013) Degradation of dipyrone in an electrochemical flow-by reactor using anodes of boron-doped diamond (BDD) supported on titanium. J Electroanal Chem 690:89–95
Malpass GRP, Neves RS, Motheo AJ (2006) A comparative study of commercial and laboratory-made Ti/Ru0.3Ti0.7O2 DSA® electrodes: “In situ” and “ex situ” surface characterisation and organic oxidation activity. Electrochim Acta 52:936–944
Motheo AJ, Gonzalez ER, Tremiliosi-Filho G, Olivi P, De Andrade AR, Kokoh B, Leger J-M, Belgsir EM, Lamy C (2000) The oxidation of formaldehyde on high overvoltage DSA type electrodes. J Braz Chem Soc 11:16–21
Gogate PR, Pandit AB (2004) A review of imperative technologies for wastewater treatment II: hybrid methods. Adv Environ Res 8:553–597
Parsons SA, Byrne A (2004) Water treatment applications. In: Parsons SA (ed) Advanced oxidation processes for water and wastewater treatment. IWA Publishing, London, pp 329–340
Shriwas AK, Gogate PR (2011) Intensification of degradation of 2,4,6-trichlorophenol using sonochemical reactors: understanding mechanism and scale-up aspects. Ind Eng Chem Res 50:9601–9608
Steter JR, Barros WRP, Lanza MRV, Motheo AJ (2014) Electrochemical and sonoelectrochemical processes applied to amaranth dye degradation. Chemosphere 17:200–207
Torres RA, Pétrier C, Combet E, Carrier M, Pulgarin C (2008) Ultrasonic cavitation applied to the treatment of bisphenol A. Effect of sonochemical parameters and analysis of BPA by-products. Ultrason Sonochem 15:605–611
Makino K, Mossoba MM, Riesz P (1982) Chemical effects of ultrasound on aqueous solutions. Evidence for hydroxyl and hydrogen free radicals (·OH and ·H) by spin trapping. J Am Chem Soc 104:3537–3539
Wu J, Liu F, Zhang H, Zhang J, Li L (2012) Decolorization of CI Reactive Black 8 by electrochemical process with/without ultrasonic irradiation. Desalin Water Treat 44:36–43
Suslick KS (1990) Sonochemistry. Science 247:1439–1445
Fitzgerald ME, Griffing V, Sullivan J (1956) Chemical effects of ultrasonics—“hot spot”‘ chemistry. J Chem Phys 25:926–933
Mason TJ, Lorimer JP, Bates DM (1992) Quantifying sonochemistry: casting some light on a ‘black art’. Ultrasonics 30:40–42
Salazar R, Garcia-Segura S, Ureta-Zañartu MS, Brillas E (2011) Degradation of disperse azo dyes from waters by solar photoelectro-Fenton. Electrochim Acta 56:6371–6379
Rodrigo MA, Michaud PA, Duo I, Panizza M, Cerisola G, Comninellis Ch (2001) Oxidation of 4-chlorophenol at boron-doped diamond electrode for wastewater treatment. J Electrochem Soc 148:D60–D64
Chai X-S, Hou QX, Luo Q, Zhu YJ (2004) Rapid determination of hydrogen peroxide in the wood pulp bleaching streams by a dual-wavelength spectroscopic method. Anal Chim Acta 507:281–284
Acknowledgments
The authors wish to thank the Brazilian research funding agencies the National Council for Scientific and Technological Development (CNPq) and the Federal Agency for the Support and Improvement of Higher Education (CAPES) for financial support. The authors also thank the BioCiTec/IQSC/USP for the liquid chromatographic determinations made in multiuser LC–MS equipment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Steter, J.R., Dionisio, D., Lanza, M.R.V. et al. Electrochemical and sonoelectrochemical processes applied to the degradation of the endocrine disruptor methyl paraben. J Appl Electrochem 44, 1317–1325 (2014). https://doi.org/10.1007/s10800-014-0742-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-014-0742-7