Abstract
The electrochemical properties of esters of p-hydroxybenzoic acid, called parabens, were investigated on a carbon fiber microelectrode and on a glassy carbon macroelectrode in glacial acetic acid containing 20 % acetonitrile (v/v) and sodium acetate as a supporting electrolyte. The anodic oxidation of parabens in this medium proceeds in a single stage giving well-shaped peaks or waves in the same potential region of above 1.0 V (vs. Ag/AgCl). The electrode process was characterized as being quasireversible, diffusion-controlled, and proceeds with the exchange of one electron and one proton. Phenoxyl radicals as products of the electrode process are chemically unstable and participate in the successive irreversible homogenous reactions resulting in electroinactive products (E q C i mechanism). Smaller oxidation potentials for parabens in comparison to such unsaturated fatty acids as oleic, linoleic, and linolenic ones indicate that these compounds can show antioxidative properties. Since parabens are often used as preservatives in many products, the results presented can be found useful in the determination of their total content in real samples.
Similar content being viewed by others
1 Introduction
Esters of p-hydroxybenzoic acid (PHB), commonly known as parabens, are extensively used as preservatives in pharmaceuticals [1–4], cosmetics [1–3, 5–9], and foodstuffs [1–3, 10–13]. Their molecular structures are shown in Scheme 1. Methyl (MP), ethyl (EP), propyl (PP), butyl (BP), and benzyl parabens (BzP) are best known. These compounds are mainly of synthetic origin. Some of them are naturally found in vegetable sources [2, 3, 14].
Parabens are recognized as ideal preservatives because of their low cost, biodegradability, good stability in air and in solutions, resistance to hydrolysis, neutral pH, lack of color, non-volatility, and thus lack of perceptible odor and taste, their broad antimicrobial spectrum (antibacterial, especially antifungal), as well as because of their relatively non-irritating and non-sensitizing properties [1–3, 15, 16]. The antimicrobial activity of parabens increases with an increase in the size of the ester group [16, 17], which results from the fact that the lipophilicity of these compounds increases in the same direction [17, 18]. This property makes it easier for parabens to get thorough the cellular membrane [17]. Since microbial replication generally occurs in the water phase of oil/water systems, the amount of parabens dissolved in water determines their preservative efficiency. In order to increase this efficiency, they are often used in combination of two or more parabens with different lipophilicity properties [1–3, 16]. Mixtures of MP and PP (less and more lipophilic, respectively) are the most commonly used preservatives because of their synergistic effect [3, 5]. As parabens are widely used in many products, humans are continuously exposed to their action.
The influence of these compounds on the human health is not totally clear. For many years, parabens have been regarded as preservatives of low toxicity. However, according to available studies, they can be harmful to the consumer due to their potential to induce allergic contact dermatitis [3, 19], they can produce inhibitory effects on mitochondrial respiratory capacities [16, 20], and they can disrupt the human reproductive potential [16, 21]. It has been suggested that the use of parabens in cosmetics may contribute to the increasing incidence of breast cancer [22–25]. Nevertheless, another study reported that parabens are safe for the human health [1–3, 5, 26–28]. Soni et al. [1] claimed that parabens are innocuous preservatives, they are practically non-toxic, non-irritating, non-sensitizing, and non-carcinogenic. The benefit/risk ratio for the use of parabens is high, and thus they must be used to guarantee the stability and safety of products. These conclusions were confirmed by Andersen in his report on the safe use of parabens in cosmetic products [5]. Nevertheless, the content of parabens in different products must be closely controlled. The use of parabens is permitted under the European Union Directives 95/2/EC (MP, EP, and PP) and 76/768/EEC (MP, EP, PP, BP, and BzP) in food and cosmetic products. They can then be used as preservatives in cosmetics up to the maximum concentration of 0.4 % for one ester and up to 0.8 % (w/w) for their mixtures, expressed as PHB. The limited use of these compounds in foodstuffs and drugs should not exceed 0.1 and 1 % (w/w), respectively [1–3]. Considering the controversial and not fully explained influence of parabens on the human health, it is very important to continue the investigations of their properties, including their electrochemical properties.
Up to now, no exact electrochemical data concerning the electrochemical properties of parabens and the mechanism of electrode reactions have been available. They were mainly examined to evaluate the optimal conditions for their determination in real samples using electrochemical detectors [8, 29–31]. Wang et al. [29] suggested a rapid and reliable electrochemical method to determine the total content of parabens in cosmetics on a GC electrode modified with polymers (MIPs) containing MP and PP as dual templates. The results described show that the parabens are oxidized on this modified electrode in phosphate-buffered aqueous solutions. The curves recorded by means of the square wave voltammetry technique consist of single peaks at the same potential of about 0.94 V (vs. SCE). These results indicate that only the total content of parabens can be calculated by this electrochemical method. Martins et al. [8] applied a boron-doped diamond electrode (BDD) as a sensor to determine parabens in shampoo by the HPLC with amperometric detection using a mobile phase consisting of water disodium phosphate solution and acetonitrile (AN). It has been shown that all parabens investigated are irreversibly oxidized at the same potential of about 0.9 V (vs. Ag/AgCl) and the overall process is diffusion controlled. This indicates that the BDD electrode is not able to differentiate the parabens. The peak potential is shifted toward positive values with a decrease in pH values, which shows that the oxidation of these compounds is more difficult in an acidic medium. Since parabens are hydrophobic compounds, the influence of AN on the electrochemical behavior of parabens was also studied. The presence of this organic solvent in aqueous solutions enhances the peak current intensity without modifying the shape of the voltammetric peaks. These results were explained in terms of enhancing the diffusion of the analyte to the surface of the working electrode. The same BDD electrode was employed by Radovan et al. [30] for the determination of parabens in hydro-alcoholic solutions by cyclic voltammetry (CV) and chronoamperometry. Since all parabens investigated (MP, EP, and PP) are oxidized in aqueous solutions containing 20 % of ethanol (v/v) giving one anodic peak at the same potential of about 0.9 V (vs. SCE), it is possible to determine only the total amount of parabens, named by the authors as “overall paraben index”. The CV curves recorded in the solutions containing PHB were very similar to those for parabens. It shows that PHB can be electrochemically detected simultaneously with the accompanying parabens. The CV experiments confirmed that the anodic oxidation of parabens in this medium proceeds irreversibly and is diffusion controlled. The similar peak potentials of the anodic oxidation of parabens were also confirmed by Kang and Kim [31]. The CV curves recorded on a GC electrode in a mixed solvent consisting of methanol and aqueous phosphoric acid showed that MP and PP are oxidized in this medium giving only one anodic peak at potentials of about 1.30 V (vs. Ag/AgCl). The existence of these peaks is due to the oxidation of the phenolic moiety in the compounds.
To the best of my knowledge, no literature data on the anodic oxidation of parabens in acidic organic solvents are available. Glacial acetic acid (HAc) is one of them. In comparison to other organic solvents, HAc has several attractive properties, such as the relatively wide potential window accessible to platinum and carbon electrodes [32], and the ability to dissolve both hydrophobic organic compounds and their matrix as well as the necessary supporting electrolyte. The disadvantageous feature of this solvent is its relatively low dielectric constant, ε (6.17 at 25 °C [33]), which results in a slight dissociation of electrolytes, and, consequently, causes significant ohmic potential drops, IR. This problem can be easily overcome by the use of microelectrodes [34–36]. As the currents recorded on microelectrodes are relatively small (pA or nA), the IR drop can be neglected even in highly resistive solutions. HAc was formerly successfully employed as a medium to investigate the electrochemical properties of hydrophobic antioxidants [37, 38] and to their voltammetric determination in pharmaceuticals [39, 40]. It seems probable that solutions of HAc can also be a good medium for anodic oxidation of parabens.
The aim of this work was to examine the anodic oxidation of parabens on carbon electrodes in HAc solutions to elucidate the mechanism of the electrode process. This paper is a logical starting point for the development of a new direct voltammetric method to be used to determine these compounds in pharmaceuticals, cosmetics, and foodstuffs.
2 Experimental
2.1 Reagents
The chemicals used were as follows: PHB, >99.0 % (Fluka, China), methyl p-hydroxybenzoate (methyl paraben, MP), ≥99.0 % (Fluka, Japan), ethyl p-hydroxybenzoate (ethyl paraben, EP), 99 % (Aldrich, Germany), propyl p-hydroxybenzoate (propyl paraben, PP), ≥99.0 %, butyl p-hydroxybenzoate (butyl paraben, BP), ≥99.0 % (each Fluka, UK), benzyl p-hydroxybenzoate (benzyl paraben, BzP), 99 % (Aldrich, USA), 3-tert-butyl-4-hydroxyanisole (BHA), ≥98 % (Fluka, Switzerland), 2,6-di-tert-butyl-p-cresol (butylated hydroxytoluene, BHT), >99.0 % (Sigma, Germany), tert-butylhydroquinone (BHQ), ≥98.0 % (Fluka, Switzerland), α-tocopherol (α-TOH), ≥96 % (Sigma, Germany), oleic acid, >99 %, linoleic acid, purriss. p.a., linolenic acid, purriss. p. a. (each Fluka, Switzerland), sodium acetate, CH3COONa (AcNa), anhydrous, ≥99.5 % (Fluka, Netherlands), sodium perchlorate, NaClO4, anhydrous, p.a. (Riedel-de Haen, Germany). AN, p.a. ahydride, HAc, p.a. ACS (each Merck, Germany) or their mixture were used as solvents in all electrochemical experiments. All reagents and solvents were of high purity and used as received.
2.2 Apparatus
Voltammetric measurements were carried out using the EA9C electrochemical analyzer connected with a Model M152 preamplifier (both MTM, Poland). The experimental conditions were controlled via a Pentium computer by a software EAGRAPH Version 4.0 (MTM, Poland). All electrochemical studies were performed using a three-electrode system: a carbon fiber (CF) disk microelectrode of 35.4-μm diameter (BASi, United Kingdom) or a glassy carbon (GC) macroelectrode of 1-mm diameter, A = 7.85 × 10−3 cm2 (Mineral, Poland) as working electrodes, a home made platinum wire auxiliary electrode, and a Ag/AgCl reference electrode with 1 M NaCl solution (Mineral, Poland). The diameter of the CF microelectrode was determined using the well-known diffusion coefficient of ferrocene in AN with 0.1 M (But)4NPF6 as a supporting electrolyte [41]. The surface of the working electrodes was mechanically polished with 0.01-μm alumina powder slurry on a polishing cloth (BASi, USA). Finally, the electrodes were sonicated in distilled water, rinsed with water and acetone, and dried before use. To avoid water leakage, the reference electrode was isolated from the test solutions by a salt bridge with a frit of Vicor Glass. In order to minimize electrical interferences, especially during the experiments with a microelectrode, the electrochemical cell was enclosed in a grounded Faraday cage.
Before voltammetric measurements, the solutions were deoxygenated by ultrasonication and then by purging with a stream of solvent–saturated argon of high purity (>99.99 %).
All measurements were carried out at room temperature (25 ± 1 °C).
A CX-732 multifunction computer meter equipped with a glass indicator electrode and Ag/AgCl reference electrode (each Elmetron, Poland) was used for pH measurements.
The specific conductivity of the solutions was measured using a Model inoLab 720 conductivity meter equipped with a standard conductivity cell TetraCon 325 (WTW, Germany). The cell constant, k, was 0.475 cm−1 (measurement range: 1 μS cm−1–2 S cm−1). The measurements were carried out at the automatic temperature compensation up to 25 °C.
Viscosity measurements were performed at 25 ± 1 °C using a ViscoClock equipped with a certified Ubbelohde capillary viscometer (the instrument constant K = 0.009872 mm2 s−2) (each SCHOTT Instruments, Germany).
3 Results and discussion
3.1 Choice of the solution composition
Preliminary investigations on the electrochemical properties of parabens consisted in selecting the appropriate solution composition. As the lipophilicity parameter (logP O/W) for these compounds increases with an increase in the size of their ester group (1.91, 2.34, 2.94, and 3.50, respectively, for MP, EP, PP, and BP [17]), and the solubility in aqueous solutions decreases, the electrochemical properties of parabens should be examined in organic solvents. The investigations on the selection of the solvent started from applying AN which is used most frequently for anodic processes [33]. Sodium perchlorate was used as a supporting electrolyte. This inexpensive electrolyte is difficult to oxidize and can expand the potential window on the positive side and can then be applied to anodic processes [33]. In order to choose the solution composition, a MP was used as a representative of the esters of PHB. The voltammetric curves of the anodic oxidation of MP in AN containing 0.1 M NaClO4 were recorded on a CF microelectrode by means of differential pulse voltammetry (DPV). The typical DPV curve is presented in Fig. 1 (curve a).
DPV voltammograms of the anodic oxidation of 0.5 mM MP recorded on a CF disk microelectrode in the solutions of: (a) AN, (b) HAc (each in the presence of 0.1 M NaClO4) or (c) in HAc and (d) in his mixture with 20 % AN (v/v) containing 0.1 M CH3COONa. DPV conditions: ΔE = 20 mV, v = 20 mV s−1, pulse width: 80 ms
MP undergoes anodic oxidation in this medium giving a main, asymmetrical peak with the maximum at the potential of about 1.6 V, and with an additional signal at that of about 1.85 V. The successively recorded curves were, however, not reproducible. The changes in peak potentials and peak currents were observed (data not shown). This is likely to result from adsorption phenomena accompanying the oxidation of MP on the surface of CF microelectrode. The better shaped main peak was observed when AN was replaced by HAc (Fig. 1, curve b). The peak potential was shifted in the negative direction in comparison with this observed in AN. This indicates that the anodic oxidation of MP proceeds easier in this medium. A decrease of the peak currents of the DPV curves recorded in HAc in comparison with those in AN can result from the higher dynamic viscosity, η of this solvent (1.130 and 0.341 mPa s at 25 °C for Hac and AN, respectively [33]) and the smaller diffusion coefficient of MP. However, the curves were still not reproducible. The reproducibility considerably increased when CH3COONa (AcNa), instead NaClO4, was used as a supporting electrolyte (curve c in Fig. 1). The peak potential, E p, was again shifted in the negative direction (from 1.42 to 1.08 V for the solutions with NaClO4 and AcNa, respectively). It is to be noticed that due to the insolubility of AcNa in AN, the DPV curves were not recorded in this medium. The peak currents of the curves recorded in HAc containing AcNa (Fig. 1, curve c) are relatively small. In order to improve the sensitivity, solutions containing a mixture of HAc and 20 % AN (v/v) and 0.1 M AcNa were examined. As can be seen from Fig. 1 (curve d), well-shaped and excellent reproducible curves were obtained with the peak potential, E p = 1.06 V. The presence of AN in the solutions tested brings about the triple increase of the peak currents. The reason for this phenomenon can be a decrease of viscosity of the mixed solvent (from 1.322 to 1.061 mPa s for HAc and its mixture with 20 % AN, respectively) and thus the increase of the diffusion coefficient for MP. These observations confirm the results obtained by Martins et al. [8] during the anodic oxidation of parabens in aqueous solutions. An addition of AN to HAc causes simultaneously a considerable increase of the specific conductivity, χ of the solutions (from 9.2 to. 250 μS cm−1). This is undoubtedly connected with the higher dielectric constant, ε, of AN in comparison with HAc (37.5 and 6.17 at 25 °C, respectively [33]) which causes an increase of the dissociation of the supporting electrolyte and prevents ion-pair formation, and, in consequence, the resistivity of the solutions diminishes. In addition, the presence of AN in HAc increased the reversibility of the anodic process. The reversibility of the electrode process can be examined using the peak width at a half height (W 1/2) of the curves recorded by DPV which can be expressed by the equation [42, 43]:
where n is the number of electrons involved in the electrode reaction. Other symbols have their usual meanings. The results obtained indicate that the values of W 1/2 for the curves recorded in HAc containing 20 % AN (v/v) drastically decreased to 0.090 V in comparison with the characteristics recorded in HAc alone (W 1/2 = 0.140 V). This first value is close to 0.0904 V at 25 °C predicted by Eq. (1) for a reversible electrode reaction which proceeds with the exchange of one electron.
The results described indicate that the mixed solvent containing HAc and AN and 0.1 M CH3COONa as a supporting electrolyte can be a good medium for the investigations of the electrochemical properties of parabens. The results previously given indicate that a larger amount of AN limited the solubility of the hydrophobic analyte, especially in the presence of a large amount of their matrix [40].
3.2 Voltammetry at microelectrodes
The investigations on the anodic oxidation of parabens in chosen medium were studied by the linear sweep voltammetry (LSV) and DPV on a CF disk microelectrode. Figure 2 (curve a) presents a typical LSV voltammetric curve recorded in the presence of MP.
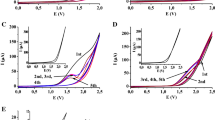
Anodic oxidation curves of 0.5 mM MP recorded by (a) LSV (v = 5 mV s−1) and (b) DPV (voltammetric parameters as in Fig. 1) on a CF microelectrode in HAc containing 20 % AN (v/v) and 0.1 M CH3COONa. Direction of polarization was reversed from anodic to cathodic at potentials E λ: 1.3, 1.5 and 1.7 V. Dashed line is residual current. Inset: semilogarithmic analysis of the curve (a)
A well-defined single anodic wave attributed to the oxidation of MP is observed. The growth of the current above electrode potential of 1.6 V is connected with the oxidation of the supporting electrolyte (dashed line on Fig. 2). This wave is situated at the potential above 1.0 V with half-wave potential, E 1/2 = 1.053 V (Table 1). When the direction of polarization was reversed from anodic to cathodic, a small hysteresis is observed. This indicates that the anodic oxidation of MP proceeds on the surface of the working electrode partially covered with products of this process. As the literature data suggest, the electrochemical oxidation of phenolic compounds can induce a passivation of the working electrode via deposition of a polymeric film on their surface which is formed by polymerization reactions of phenoxyl radicals [44–47]. Similar and negligible adsorption phenomena accompanying the anodic oxidation of parabens were also observed in hydro-alcoholic solutions [30]. In order to ensure the reproductivity of curves recorded by LSV, the surface of the CF microelectrode always needed to be polished between measurements. This procedure was not necessary when DPV was applied. This technique is considered a convenient method because of its limited influence of adsorption phenomena on recorded curves and thus excellent reproducibility, good sensibility, and selectivity, a wide range of linearity and the attainment of low quantification limit [43, 48]. The oxidation curves recorded by DPV in the same solutions consist of one well-shaped peak (curve b in Fig. 2) with peak potential, E p at 1.060 V (Table 1). This peak corresponds to the anodic wave recorded by LSV. The identical course of the voltammetric curves was obtained in solutions containing other parabens investigated: EP, PP, BP, and BzP (curves b–f in Fig. 3).
The results obtained indicate that the anodic oxidation of parabens in this medium proceeds in one stage and according to the same reaction mechanism. The size of the ester group does not affect this process. All these compounds undergo oxidation on the CF microelectrode in the same potential region. The differences between peak potentials, E p , and half-wave potentials, E 1/2. characteristic of all parabens do not exceed 0.010 V (Table 1). The reason for the anodic oxidation of parabens in the same potential region can be explained in terms of similar charge densities on the oxygen atom in the hydroxyl group of the molecules (−0.220, −0.221, −0.221, −0.221, and −0.221, respectively, for MP, EP, PP, BP, and BzP). These values were calculated by a HyperChem program with PM3 semiempirical algorithm. The results obtained show that parabens cannot be electrochemically identified in this medium. The same conclusions were drawn from the earlier electrochemical investigations [8, 29–31] (see Sect. 1).
For comparison, the voltammetric curves were also recorded in solutions containing PHB. In contrast to parabens, the anodic oxidation of PHB proceeds in two stages (Fig. 3, curve a). The first part of this curve is situated in potential region characteristic for the oxidation of parabens (curves b–f in Fig. 3) and the steady-state current is similar to that observed for its esters. The similarity of the voltammetric curves recorded in hydro-alcoholic solutions containing PHB or its esters was reported by Radovan et al. [30]. The same potential region for the anodic oxidation of these compounds can be related to comparable charge densities on the oxygen atom in the hydroxyl group for PHB (−0.219) and parabens. The second part of the curve for PHB is similar in height to the first one and is situated at the potential above 1.3 V with half-wave potential, E 1/2 = 1.40 V (Table 1). The comparable heights of these waves suggest the identical number of electrons exchanged in two stages of the electrode reaction. The DPV curve recorded in the same solution consists of two peaks: the first, well-shaped with the maximum at 1.065 V and the second one, broad and smaller at 1.390 V. The differences in the shape of these peaks can be explained in terms of the irreversibility of the second part of the anodic oxidation of PHB. In addition, the second stage of the electrode reaction proceeds probably on the surface of the working electrode partially covered by the products of the oxidation (great hysteresis in the region of the second part of the wave recorded by LSV was observed—data not shown). It should be noted that there is no signal from the oxidation of parabens in this potential region (Fig. 3).
To check the reversibility of the electrode reactions, a semi-logarithmic analysis of the curves recorded by LSV was made. According to the equation [42]:
where E (V) is the electrode potential, E 1/2 (V) is the half-wave potential, n is the number of electrons involved in the electrode reaction, I (A) is the current at a given potential E, I L (A) is the steady-state limiting current, for a reversible electrode reaction, the slope, S of the linear plot of E versus log[I/(I L−I)] in the potential range around E 1/2, expressed as dE/d(log[I/(I L−I)]) should have a value of 0.0591/n V at 25 °C. The semi-logarithmic analysis of the wave for the anodic oxidation of MP (inset in Fig. 2) is linear (r = 0.9994) with a small deviation at higher potentials. The same course of the curves was obtained for all the other parabens investigated and for PHB (data not shown). The slopes of the semi-logarithmic analysis for all parabens and for the first stage of the anodic oxidation of PHB only imperceptibly differ from the theoretic value of 0.0591 V, predicted according to Eq. (2) for one electron reversible electrode process (Table 1). These results suggest that the anodic oxidation of parabens and the first stage of this process for PHB can be related to a quasireversible exchange of one electron. In opposition to this, the slope of the second part of the semi-logarithmic analysis for PHB (0.0903 V) can correspond to the irreversible exchange of the second one-electron.
The quasireversibility of the electrode reactions confirms the Tomes criterion [49]. The differences between potentials corresponding to ¾ (E 3/4) and ¼ (E 1/4) of the steady-state current recorded by LSV does not differ much from the value of 0.0564/n V, predicted for the one-electron reversible electrode process (Table 1). As can be seen from Table 1, the deviation from the reversibility of the electrode reaction gradually increases with an increase in the size of the ester group (increased values of S and of Tomes criterion from MP to BzP). The quasireversible and one-electron character of the anodic process is also confirmed by the peak width at half height (W 1/2) of the curves recorded by DPV. The values of this parameter are close to the theoretic ones, predicted by Eq. (1) for a reversible exchange of one electron (Table 1). The higher value of W 1/2 for the second peak of the anodic oxidation of PHB confirms the irreversibility of this stage of the electrode reaction.
Assuming that the anodic oxidation of parabens and the first stage of this process for PHB proceeds with the exchange of one electron, the diffusion coefficients of these compounds were calculated from the equation which describes diffusion-limited steady state currents, I L (A) on microelectrodes [42]:
where r (m) is the radius of the electrode, n is the number of electrons involved in the electrode reaction, F is the Faraday constant, c (mol m−3) denotes the bulk concentration of the analyte, and D (m2 s−1) is the diffusion coefficient of the analyte. The values of D obtained for these compounds are listed in Table 1. As can be seen, these values only imperceptibly differ from one another and decrease with an increase in the size of the ester group.
Figure 4 shows DPV curves obtained for the oxidation of MP at different concentrations. The peak currents increased with an increase in the concentration of the analyte and no changes in peak potentials were observed.
DPV voltammograms recorded on a CF microelectrode at different concentrations of MP ranging from (a) 0.015 to (o) 0.64 mM. Dashed line is residual current. The voltammetric parameters and the solutions composition as in Fig. 1. Inset: calibration plot for MP
The plot I p versus concentration is linear in the ranges from 0.0152 mM to 0.6 mM (inset in Fig. 4). This dependence can be expressed by the linear regression equation:
where r = 0.999 for n = 18, SD of intercept: 1.1 × 10−2, SD of slope 3.2 × 10−3.
The same linear dependencies I p = f(c) and similar slopes of this relationship were observed for all the parabens investigated (data not shown). These results confirm the same number of electrons involved in the anodic oxidation of parabens, comparable values of their diffusion coefficients, and diffusion-controlled character of the electrode reaction. It is noteworthy that the peak potentials were independent of concentration and the peak currents observed were highly reproducible. The relative standard deviation (RSD) did not exceed 2 % for the ten successive recorded curves (n = 10).
The results obtained indicate that the anodic oxidation of parabens in the medium examined can apply to their determination in real samples. Since all these compounds are electrochemically undistinguishable (see Fig. 3; Table 1), only their total content can be determined and should be expressed as an amount of PHB. This procedure is recommended by the EU Directives 95/2/EC and 76/768/EEC and is described in literature [1, 3, 29, 30].
In order to check the participation of protons in the electrode reaction, the influence of pH on the E p of parabens was examined by DPV. The relative changes of pH measured in HAc containing 20 % AN (v/v) were made by the increased concentration of sodium acetate in the solutions. Acetate anions are known to be the strongest base in the HAc medium, and their increased concentration causes an increase in the pH of the solutions. In order to keep the ionic strength constant, the solutions additionally contained different amounts of NaClO4. The total concentration of the mixture was always 0.10 M. It is noteworthy that a pH scale in acetic acid (from −7 to 7.5 [33]) differs from that in water. For this reason, the abbreviation pH(HAc) was applied to the pH values measured in this medium. As the glass electrode was calibrated using aqueous buffers, the pH values can be termed as apparent pH and according to Porras and Kenndler [50] can be used to approximate relative acidities in this non-aqueous medium.
As can be seen from Fig. 5, with an increase in pH(HAc) (increased concentration of acetate anions), the peak potentials shift toward less positive values up to 1.06 V (curve e). This peak potential is characteristic for the anodic oxidation of MP in the solutions containing 0.1 M CH3COONa (Fig. 1, curve d). This indicates the participation of protons in the electrode process. The dependence between the peak potential, E p, and the pH(HAc) is linear (inset in Fig. 5) and can be expressed by the equation:
where r = 0.999 for n = 6, SD of intercept: 1.9 × 10−2, SD of slope 2.7 × 10−3.
DPV curves of the anodic oxidation of 0.12 mM MP recorded on a CF microelectrode in HAc containing 20 % AN (v/v) and different amounts of CH3COONa and NaClO4: (a) 0.01 and 0.09 M, (b) 0.04 and 0.06 M), (c) 0.06 and 0.04 M, (d) 0.08 and 0.02 M, (e) 0.10 and 0.00 M. The voltammetric parameters as in Fig. 1. Inset: relationship between peak potential, E p and pH(HAc)
The slope of this relationship is close to the expected theoretic value of 0.0591 v/pH and suggests that the equal numbers of protons and electrons are involved in the electrode reaction. The results described above indicate that the oxidation of parabens occurs with the exchange of one electron and thus with the transfer of one proton. Because parabens are weaker acids (dissociation constants, pK a equal 8.17, 8.22, 8.35, and 8.37 for MP, EP, PP, and BP, respectively [3]) in comparison to acetic acid (pK a = 4.76 [33]), they exist in this medium in a neutral form and this form undergoes an electrode reaction. The reason for decreasing the peak potential with an increase in the concentration of acetate anions in the solutions tested is a binding of protons which improves the electrode process. For pH(HAc) >1.5, the peak potential remains independent of pH (inset in Fig. 5). It can be accounted for by the fast and total binding of protons by the high excess of acetate anions existing in the solutions. The concentration of these anions above 0.08 M does not exert any influence on the equilibrium of the electrode reaction.
3.3 Cyclic voltammetry
CV gives access to additional information on the electrode process, especially about its products. These electrochemical data are not available when voltammetry on microelectrodes is used. CV experiments were carried out using a GC macroelectrode of 1.0-mm diameter. As the currents recorded on macroelectrodes are relatively high in comparison to those observed on microelectrodes, the solutions tested contained sodium acetate, the concentration of which was increased up to 0.5 M. The relatively high concentration of the salt and the presence of AN (20 %, v/v) enhanced the electric conductivity of the solutions, and thus decreased the ohmic potential drop, IR. The specific conductivity of these solutions was 1,109 μS cm−1 (about a fivefold increase in comparison to the solutions used in the experiments on CF microelectrode). Figure 6a shows a typical CV curve recorded in the potential range from −0.6 V to 1.7 V in the presence of MP (solid line) and for a supporting electrolyte alone (dashed line).
a Cyclic voltammograms of 0.53 mM MP obtained on a GC electrode in HAc containing 20 % AN (v/v) and 0.5 M CH3COONa at 100 mV s−1 with different switching potentials E λ: 1.07, 1.30, 1.50, and 1.70 V. Dashed line is residual current. b Comparison of the CV curves for MP (c = 0.53 mM), EP (c = 0.48 mM), PP (c = 0.52 mM), BP (c = 0.50 mM), and BzP (c = 0.52 mM)
As can be seen, a single-stage oxidation of MP occurs, which results in a well-shaped peak at the potential of about 1.05 V. The position of this peak corresponds with the wave and peak observed on CV and DPV curves recorded on a CF microelectrode (Fig. 2). An unlimited increase of the current above 1.6 V results in the oxidation of the supporting electrolyte (dashed line in Fig. 6a). When the direction of polarization was reversed from anodic to cathodic, no reduction peaks were recorded even when the direction of polarization was reversed at the potential E λ near the anodic oxidation peak. The loss of the current peak on the reverse scan of a cyclic voltammogram is the most obvious indicator of a homogenous chemical reaction step consuming the product generated on the surface of the working electrode [42]. The absence of a reduction peak indicates that the products of the anodic oxidation of MP are very unstable and participate in the successive irreversible homogenous reaction. A similar course of CV curves was also observed for other parabens investigated: EP, PP, BP, and BzP (Fig. 6b). These observations confirm the earlier assumptions that the size of the ester group exerts no influence on the mechanism of the electrode process.
On recording successive scans in the same solution, without cleaning the electrode surface, a small and gradual decrease in peak currents was observed (data not shown). This confirms the fact that the adsorption phenomena accompanying the oxidation of parabens on a GC reduce the available electrode surface area. A 15 % decrease in the peak current was obtained from the first to the tenth scan for all the parabens investigated. The largest changes were observed between the first and the second cycle (about 8 % change). The peaks in the next cycles gradually achieved a stable height. Since the anodic oxidation of parabens is diffusion controlled, the decrease in peak current probably results from the partial adsorption of the final products of the electrode process [44–47]. A similar decrease of the anodic peak current corresponding to the oxidation of parabens on BDD electrode in hydro-alcoholic solutions [30] and of PHB on a GC electrode in phosphate buffer solutions [47] was observed. This phenomenon can be explained in terms of the electrode fouling caused by the electro-oxidized product. It should be stressed that no additional peaks were observed upon repetitive cycling. Between measurements, the electrode was always polished to avoid possible problems from the adsorption of oxidation products on a GC surface.
In order to check whether the process occurring on a GC electrode is under diffusion or adsorption control, the scan rate studies were made. The height of the anodic peaks for all the parabens investigated increased with an increase in the potential scan rate, v (Fig. 7).
CV curves obtained on a GC electrode in solutions containing 0.48 mM EP at different scan rates: (a) 10, (b) 25, (c) 50, (d) 100, and (e) 200 mV s−1. Other components of the test solutions as in Fig. 6. Inset: relationship between anodic peak currents, I p, and square root of scan rate, v 1/2
According to the Randles-Sevcik equation, the dependence of the peak current, I p, on v 1/2 for diffusion-controlled process should be linear. The nature of this dependence for EP is linear (inset in Fig. 7) in the range of 5–250 mV s−1 and can be expressed by the equation:
where r = 0.998 for n = 8, SD of intercept: 6.7 × 10−3, SD of slope 4.2 × 10−3.
Very similar relationships were obtained for all the parabens investigated. The results obtained confirm that the anodic oxidation of these compounds is diffusion controlled and the adsorption phenomena accompanying the anodic oxidation of parabens can be related to the adsorption of the products. Moreover, the peak potentials shifted positive with an increase in the scan rate (Fig. 7). This can result from the quasireversible character of the electrode reaction, certified on the basis of the results obtained using a CF microelectrode. The quasireversible character of the anodic process confirms the width of the anodic peaks (δE pa = E pa− E pa/2), where E pa is the potential of anodic peak and E pa/2 is the potential at the half height of the peak. The values of δE pa for parabens investigated only imperceptibly differ from those expected for a reversible one-electron transfer, i.e., 0.0565 V [42]. Thus, with v = 5–100 mV s−1, δE pa was between 0.0605 and 0.081 V. These results indicate that the oxidation of parabens is not strictly Nernstian.
An analysis of the data taken from CV experiments indicates that the peak potential, E p, varies linearly with the logarithm of the scan rate, v. The plot of E p versus logv (not shown) was linear and for MP it can be expressed by the equation:
where r = 0.990 for n = 5, SD of intercept: 5.1 × 10−2, SD of slope 2.2 × 10−3.
The slope of this curve (0.0325 V dec−1) is similar to those obtained for EP, PP, BP, and BzP (0.0308, 0.0307, 0.0295, and 0.0305 V dec−1, respectively). These results are consistent with the EC behavior in which the charge transfer is followed by a chemical followup step. The slopes of the curves are very close to the theoretically expected value of 0.030/n per tenfold increase in v for the first order chemical reaction [42, 51].
3.4 Mechanism of the anodic oxidation of parabens
The results presented show that the anodic oxidation of parabens on carbon electrodes in HAc containing 20 % AN (v/v) and CH3COONa as a supporting electrolyte proceeds with the exchange of one electron and one proton. Since parabens are weaker acids in comparison to acetic acid (see Sect. 3.2), they exist in this medium in a neutral form and this form undergoes an electrode reaction. It seems to be clear that this oxidation reaction occurs on the OH group in position four of the aromatic ring. With regards to the similarity of charge densities on the oxygen atom in this group (see Sect. 3.2) and thus to the similarity of the curves recorded in the presence of all parabens (Fig. 3, 6b), the anodic oxidation of these compounds proceeds according to the same mechanism. A tentative mechanism for the anodic oxidation of parabens is presented in Scheme 2.
The loss of the electron is quasireversible in character and produces a phenoxyl radical. The formation of such a product in the anodic oxidation of phenol and its derivatives has been described in literature [44–46]. The increased concentration of CH3COO− ions in the solutions tested improves the electrode process by binding the protons, and thus moving the equilibrium of this reaction in the direction of products. Consequently, a decrease in the potentials of the oxidation of parabens was observed (Fig. 5). The results obtained with the use of CV indicate that a phenoxyl radical is chemically unstable and participates in the successive homogenous reaction. Consequently, no reduction peaks were observed. The nature of this chemically irreversible reaction is not fully clear. As described above, this chemical reaction is of first order and it seems probable that the phenoxyl radical formed upon the heterogenous electron transfer can react with a molecule of paraben to yield a predominantly para-linked dimeric radical. Subsequent reactions produce neutral dimers and oligomers which can form a passivating film on the surface of a working electrode. Such a mechanism for the anodic oxidation of the phenolic compounds in aqueous solutions has been described in the literature [44, 45].
It is noteworthy that the mechanism of the anodic oxidation of PHB differs from that described for parabens. The phenoxyl radical formed at the first one-electron quasireversible stage of this electrode process for PHB is probably more stable in comparison with that characteristic of parabens and can then undergo a second irreversible exchange of electron (Fig. 3, curve a) giving a quinone derivative as a final product of their oxidation. This oxidation product of PHB was postulated by Hu et al. [47].
Assuming the results obtained, it can be stressed that the anodic oxidation of parabens on carbon electrodes in HAc containing 20 % AN (v/v) and CH3COONa as a supporting electrolyte proceeds according to the E q C i mechanism. It is noteworthy that it is only one of the probable mechanisms of this process. In our laboratory, we could not identify primary and final products formed during the anodic oxidation of parabens.
3.5 Antioxidative properties of parabens
The antioxidative properties of compounds can be successfully investigated by the voltammetric techniques. This method is based on the measurement of the half-wave potential (E 1/2) or peak potential (E p) in a given medium, which should be inversely proportional to the antioxidant power, i.e., the lower E 1/2 or E p, the higher the antioxidative activity [52, 53]. In order to verify direct antioxidative properties of parabens, the E p values of the curves recorded by DPV for these compounds were compared with those characteristic of common antioxidants: the natural α-tocopherol (α-TOH), synthetic (BHA, BHT, and BHQ) and the essential fatty acids with three, two, and one double bonds: linolenic, linoleic, and oleic (Fig. 8).
a Anodic oxidation curves recorded by DPV on a CF microelectrode in Hac containing 20 % AN (v/v), 0.1 M CH3COONa and (a) α-TOH, (b) BHQ, (c) BHA, (d) MP, (e) BHT (each in concentration of 0.5 mM), unsaturated fatty acids: (f) linolenic, (g) linoleic, and (h) oleic (each in concentration of 1. 0 mM). The curves for fatty acids were recorded in the presence of 0.1 M NaClO4 . Dashed and dotted lines are residual currents for CH3COONa and NaClO4, respectively. Voltammetric parameters as in Fig. 1. b Antioxidant power of parabens in comparison with other natural and synthetic antioxidants expressed as peak potential, E p, of their anodic oxidation curves
Since the potential windows of the solutions containing CH3COONa as a supporting electrolyte is shorter in comparison with that containing NaClO4 (dashed and dotted lines in Fig. 8a, respectively), the curves for the unsaturated fatty acids were recorded in the presence of sodium perchlorate. As the parabens are oxidized at the same potentials (Fig. 3), for the clarity of the picture, only the curve for MP was presented in Fig. 8a (curve d). The parabens are oxidized at smaller potentials in comparison with unsaturated fatty acids, and thus they can protect these compounds against oxidative damage in a direct action. Antioxidative properties of parabens are not so good as these of α-tocopherol—one of the best known natural hydrophobic antioxidant (curve a in Fig. 8a) and synthetic antioxidants: BHQ and BHA (curves b and c). The antioxidative power of parabens is, however, higher in comparison with other synthetic antioxidant BHT. A comparison of the peak potentials characteristic of these antioxidants and of unsaturated fatty acids is presented in Fig. 8b. As can be seen, the antioxidative properties of all the parabens investigated are very similar (similar peak potentials of their DPV oxidation curves). Comparable results were obtained by Krotkova et al. [18] by a method based on electroreduction of oxygen in the presence of parabens in phosphate buffer solutions.
The results obtained indicate that parabens can be used not only as antimicrobial preservatives but also for the oxidative stability of pharmaceuticals, cosmetics, and foodstuffs as well.
4 Conclusions
The results obtained show that HAc containing 20 % AN (v/v) and CH3COONa as a supporting electrolyte can be a good medium for the investigations of the electrochemical properties of parabens. The anodic oxidation of parabens in this medium proceeds in one stage giving well-shaped voltammetric signals in the same potential region. This indicates that the size of the ester group does not affect the mechanism of the electrode reaction. This electrode process was characterized as being quasireversible, diffusion controlled, and proceeds with the exchange of one electron and one proton. The most probable product of the anodic reaction is phenoxyl radical which is chemically unstable and participates in the successive irreversible homogenous reactions. These subsequent reactions produce neutral dimers and oligomers which form a passivating film on the surface of a working electrode. Thus, the anodic oxidation of parabens on carbon electrodes in the examined medium proceeds according to the E q C i mechanism.
Very good reproducibility of peak potentials and peak currents of the curves recorded by DPV indicates that the anodic oxidation of parabens can be used in the determination of this compound in real samples. Since the parabens investigated are electrochemically undistinguishable, only their total content can be determined. Smaller potentials of the oxidation of parabens in comparison with those of unsaturated fatty acids indicate that these compounds act simultaneously as antimicrobial agents and antioxidants and can be useful in the oxidative stability of pharmaceuticals, cosmetics, and foodstuffs.
References
Soni MG, Budrock GA, Taylor SL, Greenberg NA (2001) Safety assessment of propyl paraben: a review of published literature. Food Chem Toxicol 39:513–532
Soni MG, Taylor SL, Greenberg NA, Budrock GA (2002) Evaluation of the health aspects of methyl paraben: a review of the published literature. Food Chem Toxicol 40:1335–1373
Soni MG, Carabin IG, Budrock GA (2005) Safety assessment of esters of p-hydroxybenzoic acid (parabens). Food Chem Toxicol 43:985–1015
Baalbaki B, Blanchin M-D, Fabre H (2002) Validation of a micellar electrokinetic capillary chromatography method for the determination of imidurea, methyl and propylparabens in a pharmaceutical ointment. Anal Chim Acta 463:15–20
Andersen FA (2008) Final amended report on the safety assessment of methylparaben, ethylparaben, propylparaben, isopropylparaben, butylparaben, isobutylparaben, and benzylparaben as used in cosmetic products. Int J Toxicol 27(Suppl 4):1–82
Marquez-Sillero I, Aguilera-Herrador E, Cardenas S, Valcarcel M (2010) Determination of parabens in cosmetic products using multi-walled carbon nanotubes as solid phase extraction sorbent and corona-charged aerosol detection system. J Chromatogr A 1217:1–6
Gao W, Legido-Quibley C (2011) Fast and sensitive high performance liquid chromatography analysis of cosmetic creams for hydroquinone, phenol and six preservatives. J Chromatogr A 1218:4307–4311
Martins I, Carreira FC, Canaes LS, de Souza Campos FA Jr, da Silva Cruz LM, Rath S (2011) Determination of parabens in shampoo using high performance liquid chromatography with amperometric detection on a boron-doped diamond electrode. Talanta 85:1–7
Murakami K, Watanabe H, Tateno T, Kauffmann J-M (2010) Comparative study of the determination of parabens in shampoos by liquid chromatography with amperometric and coulometric detection. Electroanalysis 22:1702–1706
Myint A, Zhang Q, Liu L, Cui H (2004) Flow injection-chemiluminescence determination of paraben preservative in food safety. Anal Chim Acta 517:119–124
Wang L, Zhang X, Wang Y, Wang W (2006) Simultaneous determination of preservatives in soft drinks, yogurts and sauces by a novel solid-phase extraction element and thermal desorption-gas chromatography. Anal Chim Acta 577:62–67
Wang W, Wang Y, Zhang J, Chu Q, Ye J (2010) Simultaneous determination of electroactive and non-electroactive food preservatives by novel capillary electrophoresis with amperometric detection. Anal Chim Acta 678:39–43
Saad B, MdF Bari, Saleh MI, Ahmad K, Talib MKM (2005) Simultaneous determination of preservatives (benzoic acid, sorbic acid, methylparaben and propylparaben) in foodstuffs using high-performance liquid chromatography. J Chromatogr A 1073:393–397
Bais HP, Vepachedu R, Vivanco JM (2003) Root specific elicitation and exudation of fluorescent β-carbolines in transformed root cultures of Oxalis tuberosa. Plant Physiol Biochem 41:345–353
Sznitowska M, Janicki S, Dabrowska EA, Gajewska M (2002) Physicochemical screening of antimicrobial agents as potential preservatives for submicron emulsions. Eur J Pharm Sci 15:489–495
Tavares RS, Martins FC, Oliveira PJ, Ramalho-Santos J, Peixoto FP (2009) Parabens in male infertility-Is there a mitochondrial connection? Reprod Toxicol 27:1–7
Angelov T, Vlasenko A, Tashkov W (2008) HPLC determination of pKa of parabens and investigation on their lipophilicity parameters. J Liq Chromatogr Relat Technol 31:188–197
Korotkova EI, Avramchik OA, Angelov TM, Karbainov YA (2005) Investigation of antioxidant activity and lipophilicity parameters of some preservatives. Electrochim Acta 51:324–332
Furrer P, Mayer JM, Gurny R (2002) Ocular tolerance of preservatives and alternatives. Eur J Pharm Biopharm 53:263–280
Martins F, Margarida A, Oliveira MM, Oliveira PJ, Peixoto FP (2008) Toxicity of parabens in testis mitochondria: a possible role on male infertility. Biochim Biophys Acta Bioenerg 1777:S60–S61
Chen J, KCh Ahn, Gee NA, Gee SJ, Hammock BD, Lasley BL (2007) Antiandrogenic properties of parabens and other phenolic containing small molecules in personal care products. Toxicol Appl Pharmacol 221:278–284
Darbre PD, Harvey PW (2008) Parabens esters: review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks. J Appl Toxicol 28:561–578
McGrath KG (2003) An earlier age of breast cancer diagnosis related to more frequent use of antiperspirants/deodorants and underarm shaving. Eur J Cancer Prev 12:479–485
Shanmugam G, Ramaswamy BR, Radhakrishnan V, Tao H (2010) GC-MS method for the determination of paraben preservatives in the human breast cancerous tissue. Microchem J 96:391–396
Mirick DK, Davis S, Thomas DB (2002) Antiperspirant use and the risk of breast cancer. J Natl Cancer Inst 94:1578–1580
ChJ Rageth (2005) One step forward in proving, that underarm cosmetics do not cause breast cancer. Breast 14:85–86
Epstein H (2006) Cosmetics preservation: sense and nonsense. Clin Dermatol 24:551–552
Scialli AR (2011) Reproductive effects of the parabens. Reprod Toxicol 32:138–140
Wang Y, Cao Y, Fang Ch, Gong Q (2010) Electrochemical sensor for parabens based on molecular imprinting polymers with dual-templates. Anal Chim Acta 673:145–150
Radovan C, Cinghita D, Manea F, Mincea M, Cofan C, Ostafe V (2008) Electrochemical sensing and assessment of parabens in hydro-alcoholic solutions and water using a boron-doped diamond electrode. Sensors 8:4330–4349
Kang SH, Kim H (1997) Simultaneous determination of methylparaben, propylparaben and thimerosal by high-performance liquid chromatography and electrochemical detection. J Pharm Biomed Anal 15:1359–1364
Michalkiewicz S, Kaczor M (2004) Accessible potential windows of platinum and carbon electrodes in acetic acid and its mixtures with ethyl acetate. Chem Anal (Warsaw) 49:121–128
Izutsu K (2002) Electrochemistry in nonaqueous solutions. Wiley–VCH, Weinheim
Brett CMA, Brett AMO (1998) Electroanalysis. Oxford University Press, New York
Stojek Z (1991) Mikrochim. Acta (Wien). New possibilities in analytical chemistry connected with voltammetric applications of microelectrodes II:353–361
Bond AM (1994) Past, present and future contributions of microelectrodes to analytical studies employing voltammetric detection. Analyst 119:R1–R21
Michalkiewicz S, Tutaj M, Kaczor M, Malyszko J (2002) Electrochemical behavior of tocopherols on microelectrodes in acetic acid medium. Electroanalysis 14:297–302
Michalkiewicz S (2011) Anodic oxidation of oxidized forms of coenzymes Q10 and Q0 on carbon electrodes in acetic acid solutions. Bioelectrochemistry 82:103–111
Michalkiewicz S, Pryciak M, Malyszko J, Oszczudlowski J (2004) Voltammetric determination of α-tocopheryl acetate in pharmaceutical dosage forms. Electroanalysis 16:961–965
Michalkiewicz S (2008) Voltammetric determination of coenzyme Q10 in pharmaceutical dosage forms. Bioelectrochemistry 73:30–36
Hyk W, Stojek Z (1997) Chronoamperometry of uncharged species under the conditions of deficiency of supporting electrolyte: experiment versus theory. J Electroanal Chem 422:179–194
Sholz F (ed) (2002) Electroanalytical methods. Guide to experiments and applications. Springer, Berlin
Bard AJ, Faulkner LR (2001) Electrochemical methods. Fundamentals and applications. Wiley, New York
Wang J, Jiang M, Lu F (1998) Electrochemical quartz crystal microbalance investigation of surface fouling due to phenol oxidation. J Electroanal Chem 444:127–132
Ferreira M, Varela H, Torresi RM, Tremiliosi-Filho G (2006) Electrode passivation caused by polymerization of different phenolic compounds. Electrochim Acta 52:434–442
Ureta-Zanartu MS, Bustos P, Berrios C, Diez MC, Mora ML, Gutierrez C (2002) Electrooxidation of 2, 4-dichlorophenol and other polychlorinated phenols at a glassy carbon electrode. Electrochim Acta 47:2399–2406
Hu Y-L, Lu Y, Zhou G-J, Xia X-H (2008) A simple electrochemical method for the determination of hydroxyl free radicals without separation process. Talanta 74:760–765
Blanc R, Gonzales-Casado A, Navalon A, Vilchez JL (2000) On the estimate of blanks in differential pulse voltammetric techniques: application to detection limits evaluation as recommended by IUPAC. Anal Chim Acta 403:117–123
Bond AM, Oldham KB, Zoski CG (1989) Steady-state voltammetry. Anal Chim Acta 216:177–230
Porras SP, Kenndler E (2004) Capillary zone electrophoresis in non-aqueous solutions: pH of the background electrolyte. J Chromatogr A 1037:455–465
Greef R, Peat R, Peter LM, Pletchner D, Robinson J (1985) Instrumental methods in electrochemistry. Wiley, New York
Chevion S, Roberts MA, Chevion M (2000) The use of cyclic voltammetry for the evaluation of antioxidant capacity. Free Radic Biol Med 28:860–870
Yang B, Kotani A, Arai K, Kusu F (2001) Estimation of the antioxidant activities of flavonoids from their oxidation potentials. Anal Sci 17:599–604
Acknowledgments
We owe our gratitude to Mrs Agata Skorupa for her technical assistance.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Michalkiewicz, S. Anodic oxidation of parabens in acetic acid–acetonitrile solutions. J Appl Electrochem 43, 85–97 (2013). https://doi.org/10.1007/s10800-012-0502-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-012-0502-5