Abstract
Purpose
To compare the efficacy of intravitreal ranibizumab between non-vitrectomized and vitrectomized eyes with diabetic macular edema (DME).
Study design
A retrospective, nonrandomized, and comparative study.
Methods
From May 2013 to March 2016, 148 eyes of 148 patients with treatment-naïve center-involving DME were reviewed in one institution. Forty-six eyes underwent prior vitrectomy at least 3 months ago, and 102 eyes did not receive any vitrectomy. Three monthly then PRN intravitreal ranibizumab treatments were performed in all the patients with monthly follow-up for 6 months. Primary outcome measures included change in central foveal thickness (CFT) and best-corrected visual acuity (BCVA) at month 6.
Results
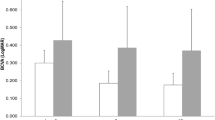
The CFT significantly reduced, and the BCVA significantly improved 6 months after ranibizumab injections in either vitrectomized or non-vitrectomized groups (p < 0.05). There was no difference between vitrectomized and non-vitrectomized eyes in baseline characteristics. Significantly better final BCVA and visual gain were found in non-vitrectomized eyes than in vitrectomized eyes (p = 0.01 and 0.03, respectively). Final CFT and CFT decrease were significantly greater in non-vitrectomized group than in vitrectomized group (p = 0.02 and 0.006, respectively). Injection number of ranibizumab was 4.12 ± 0.58 in non-vitrectomized eyes, significantly less than that in vitrectomized eyes (5.05 ± 0.71) during 6-month period (p < 0.001). There were no severe systemic/ocular adverse effects in both groups.
Conclusions
Intravitreal ranibizumab was helpful for either vitrectomized or non-vitrectomized eyes with DME in short-term follow-up. Anatomical and functional improvements were greater in non-vitrectomized patients than in vitrectomized cases.


Similar content being viewed by others
References
Ferrara N (2004) Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 25:581–611
Funatsu H, Yamashita H, Sakata K, Noma H, Mimura T, Suzuki M, Eguchi S, Hori S (2005) Vitreous levels of vascular endothelial growth factor and intracellular adhesion molecule 1 are related to diabetic macular edema. Ophthalmology 112:806–816
Brown DM, Nguyen QD, Marcus DM, Boyer DS, Patel S, Feiner L, Schlottmann PG, Rundle AC, Zhang J, Rubio RG, Adamis AP, Ehrlich JS, Hopkins JJ, RIDE and RISE Research Group (2013) Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology 120:2013–2022
Schmidt-Erfurth U, Lang GE, Holz FG, Schlingemann RO, Lanzetta P, Massin P, Gerstner O, Bouazza AS, Shen H, Osborne A, Mitchell P, RESTORE Extension Study Group (2014) Three-year outcomes of individualized ranibizumab treatment in patients with diabetic macular edema: the RESTORE extension study. Ophthalmology 121:1045–1053
Bressler SB, Glassman AR, Almukhtar T, Bressler NM, Ferris FL, Googe JM Jr, Gupta SK, Jampol LM, Melia M, Wells JA, Diabetic Retinopathy Clinical Research Network (DRCR.net) (2016) Five-year outcomes of ranibizumab with prompt or deferred laser versus laser or triamcinolone plus deferred ranibizumab for diabetic macular edema. Am J Ophthalmol 164:57–68
Rajendram R, Fraser-Bell S, Kaines A, Michaelides M, Hamilton RD, Esposti SD, Peto T, Egan C, Bunce C, Leslie RD, Hykin PG (2012) A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24-month data: report 3. Arch Ophthalmol 130:972–979
Kakinoki M, Sawada O, Sawada T, Saishin Y, Kawamura H, Ohji M (2012) Effect of vitrectomy on aqueous VEGF concentration and pharmacokinetics of bevacizumab in macaque monkeys. Invest Ophthalmol Vis Sci 53:5877–5880
Chin HS, Park TS, Moon YS, Oh JH (2005) Difference in clearance of intravitreal triamcinolone acetonide between vitrectomized and nonvitrectomized eyes. Retina 25:556–560
Christoforidis JB, Williams MM, Wang J, Jiang A, Pratt C, Abdel-Rasoul M, Hinkle GH, Knopp MV (2013) Anatomic and pharmacokinetic properties of intravitreal bevacizumab and ranibizumab after vitrectomy and lensectomy. Retina 33:946–952
Beer PM, Bakri SJ, Singh RJ, Liu W, Peters GB 3rd, Miller M (2003) Intraocular concentration and pharmacokinetics of triamcinolone acetonide after a single intravitreal injection. Ophthalmology 110:681–686
Ahn SJ, Ahn J, Park S, Kim H, Hwang DJ, Park JH, Park JY, Chung JY, Park KH, Woo SJ (2014) Intraocular pharmacokinetics of ranibizumab in vitrectomized versus nonvitrectomized eyes. Invest Ophthalmol Vis Sci 55:567–573
Ahn J, Kim H, Woo SJ, Park JH, Park S, Hwang DJ, Park KH (2013) Pharmacokinetics of intravitreally injected bevacizumab in vitrectomized eyes. J Ocul Pharmacol Ther 29:612–618
Yanyali A, Aytug B, Horozoglu F, Nohutcu AF (2007) Bevacizumab (Avastin) for diabetic macular edema in previously vitrectomized eyes. Am J Ophthalmol 144:124–126
Kondo M, Ito Y, Terasaki H (2007) Intravitreal bevacizumab (Avastin) for treatment of persistent macular edema in vitrectomized eyes: limited effect and early recurrence. Retina Cases Brief Rep 1:195–197
Okamoto Y, Okamoto F, Hiraoka T, Oshika T (2014) Vision-related quality of life and visual function following intravitreal bevacizumab injection for persistent diabetic macular edema after vitrectomy. Jpn J Ophthalmol 58:369–374
Bressler SB, Melia M, Glassman AR, Almukhtar T, Jampol LM, Shami M, Berger BB, Bressler NM, Diabetic Retinopathy Clinical Research Network (2015) Ranibizumab plus prompt or deferred laser for diabetic macular edema eyes with vitrectomy before anti-vascular endothelial growth factor therapy. Retina 35:2516–2528
Early Treatment Diabetic Retinopathy Study Research Group (1985) Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol 103:1796–1806
Acknowledgements
The study did not have any government support. This study was supported by Grants of Far Eastern Memorial Hospital (FEMH - 2016-C-044), Taipei.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no proprietary or commercial interest in any materials discussed in this article. The authors declare no financial support or conflicts of interest.
Rights and permissions
About this article
Cite this article
Chen, YY., Chen, PY., Chen, FT. et al. Comparison of efficacy of intravitreal ranibizumab between non-vitrectomized and vitrectomized eyes with diabetic macular edema. Int Ophthalmol 38, 293–299 (2018). https://doi.org/10.1007/s10792-017-0462-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-017-0462-1