Abstract
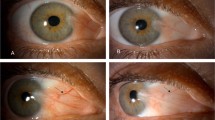
The present study was undertaken to compare the pterygium recurrence rates after treatment with two different concentrations of topical bevacizumab in those who had undergone a primary pterygium excision. The 90 patients who underwent pterygium excision were enrolled in this prospective, placebo-controlled double-blinded interventional case series. The participants were randomly categorized into 3 groups each consisting of 30 subjects. 24 h after surgery, Group II and Group III received a total of 5 and 10 mg/mL dose of topical bevacizumab, respectively; whereas patients in Group I were administered only a placebo starting a day after surgery. Participants were instructed to instill their topical medicines 4 times a day for 1 week. The patients were examined for pterygium recurrence and complications at postoperative 1, 7, and 14 days as well as each month during the following year. Pterygia recurred in 14 patients (46.7 %) in Group I and in 4 patients (13.3 %) in Group II. No recurrence was observed in Group III during the follow-up period. The Kaplan–Meier survival analysis disclosed a significantly better outcome for those who had been treated with 10 mg/mL concentrations of bevacizumab (Mantel–Cox log rank analysis, P < 0.001). The mean recurrence time was not significantly different between Group I and Group II. No ocular or systemic complication developed till the end of follow-up. Thus, 10 mg/mL concentration of topical bevacizumab was more efficacious than 5 mg/mL dose in preventing pterygium recurrence.

Similar content being viewed by others
References
Farrah JJ, Lee GA, Greenrod E, Vieira J (2006) Outcomes of autoconjunctival grafting for primary pterygia when performed by consultant compared with trainee ophthalmologists. Clin Exp Ophthalmol 34(9):857–860. doi:10.1111/j.1442-9071.2006.01341.x
Pinkerton OD, Hokama Y, Shigemura LA (1984) Immunologic basis for the pathogenesis of pterygium. Am J Ophthalmol 98(2):225–228
Papadia M, Barabino S, Valente C, Rolando M (2008) Anatomical and immunological changes of the cornea in patients with pterygium. Curr Eye Res 33(5):429–434. doi:10.1080/02713680802130354
Chui J, Di Girolamo N, Wakefield D, Coroneo MT (2008) The pathogenesis of pterygium: current concepts and their therapeutic implications. Ocul Surf 6(1):24–43
Mauro J, Foster CS (2009) Pterygia: pathogenesis and the role of subconjunctival bevacizumab in treatment. Semin Ophthalmol 24(3):130–134. doi:10.1080/08820530902801106
Adamis AP, Shima DT (2005) The role of vascular endothelial growth factor in ocular health and disease. Retina 25(2):111–118
Hosseini H, Nejabat M, Khalili MR (2007) Bevacizumab (Avastin) as a potential novel adjunct in the management of pterygia. Med Hypotheses 69(4):925–927. doi:10.1016/j.mehy.2007.01.047
Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z (1999) Vascular endothelial growth factor (VEGF) and its receptors. FASEB J 13(1):9–22
Stevenson W, Cheng SF, Dastjerdi MH, Ferrari G, Dana R (2012) Corneal neovascularization and the utility of topical VEGF inhibition: ranibizumab (Lucentis) vs bevacizumab (Avastin). Ocul Surf 10(2):67–83. doi:10.1016/j.jtos.2012.01.005
Galor A, Yoo SH, Piccoli FV, Schmitt AJ, Chang V, Perez VL (2010) Phase I study of subconjunctival ranibizumab in patients with primary pterygium undergoing pterygium surgery. Am J Ophthalmol 149(6):926–931. doi:10.1016/j.ajo.2010.01.015
Ferrara N, Hillan KJ, Novotny W (2005) Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun 333(2):328–335. doi:10.1016/j.bbrc.2005.05.132
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350(23):2335–2342. doi:10.1056/NEJMoa032691
Leippi S, Grehn F, Geerling G (2009) Antiangiogenic therapy for pterygium recurrence. Ophthalmologe 106(5):413–419. doi:10.1007/s00347-009-1936-y
Ozgurhan EB, Agca A, Kara N, Yuksel K, Demircan A, Demirok A (2013) Topical application of bevacizumab as an adjunct to recurrent pterygium surgery. Cornea 32(6):835–838. doi:10.1097/ICO.0b013e3182772d4e
Wu PC, Kuo HK, Tai MH, Shin SJ (2009) Topical bevacizumab eyedrops for limbal-conjunctival neovascularization in impending recurrent pterygium. Cornea 28(1):103–104. doi:10.1097/ICO.0b013e3181822615
Sanchez-Thorin JC, Rocha G, Yelin JB (1998) Meta-analysis on the recurrence rates after bare sclera resection with and without mitomycin C use and conjunctival autograft placement in surgery for primary pterygium. Br J Ophthalmol 82(6):661–665
Fallah MR, Khosravi K, Hashemian MN, Beheshtnezhad AH, Rajabi MT, Gohari M (2010) Efficacy of topical bevacizumab for inhibiting growth of impending recurrent pterygium. Curr Eye Res 35(1):17–22. doi:10.3109/02713680903395273
D’Ombrain A (1948) The surgical treatment of pterygium. Br J Ophthalmol 32(2):65–71
Andreoli CM, Miller JW (2007) Anti-vascular endothelial growth factor therapy for ocular neovascular disease. Curr Opin Ophthalmol 18(6):502–508. doi:10.1097/ICU.0b013e3282f0ca54
Manayath GJ, Narendran V, Al-Kharousi N, Wali UK (2009) Bevacizumab therapy for macular edema in central retinal vein occlusion: long-term results. Oman J Ophthalmol 2(2):73–78. doi:10.4103/0974-620X.53036
Jardeleza MS, Miller JW (2009) Review of anti-VEGF therapy in proliferative diabetic retinopathy. Semin Ophthalmol 24(2):87–92. doi:10.1080/08820530902800330
El-Mollayess GM, Noureddine BN, Bashshur ZF (2011) Bevacizumab and neovascular age related macular degeneration: pathogenesis and treatment. Semin Ophthalmol 26(3):69–76. doi:10.3109/08820538.2010.545100
Martinez-Carpio PA, Bonafonte-Marquez E, Heredia-Garcia CD, Bonafonte-Royo S (2008) Efficacy and safety of intravitreal injection of bevacizumab in the treatment of neovascular glaucoma: systematic review. Arch Soc Esp Oftalmol 83(10):579–588
Bock F, Konig Y, Kruse F, Baier M, Cursiefen C (2008) Bevacizumab (Avastin) eye drops inhibit corneal neovascularization. Graefes Arch Clin Exp Ophthalmol 246(2):281–284. doi:10.1007/s00417-007-0684-4
Chen JJ, Ebmeier SE, Sutherland WM, Ghazi NG (2011) Potential penetration of topical ranibizumab (Lucentis) in the rabbit eye. Eye (Lond) 25(11):1504–1511. doi:10.1038/eye.2011.225
Karalezli A, Kucukerdonmez C, Akova YA, Koktekir BE (2014) Does topical bevacizumab prevent postoperative recurrence after pterygium surgery with conjunctival autografting? Int J Ophthalmol 7(3):512–516. doi:10.3980/j.issn.2222-3959.2014.03.23
Sudhalkar A, Sudhalkar A (2012) Outcomes of post-operative topical bevacizumab in primary pterygium surgery: a case series. J Clin Exp Ophthalmol 3(7):243. doi:10.4172/2155-9570.1000243
Alhammami H, Farhood Q, Shuber H (2013) Subconjunctival Bevacizumab Injection in Treatment of recurrent Pterygium. J Clin Exp Ophthal 4(267):2. doi:10.4172/2155-9570.1000267
Nava-Castaneda A, Olvera-Morales O, Ramos-Castellon C, Garnica-Hayashi L, Garfias Y (2014) Randomized, controlled trial of conjunctival autografting combined with subconjunctival bevacizumab for primary pterygium treatment: 1 year follow-up. Clin Exp Ophthalmol 42(3):235–241. doi:10.1111/ceo.12140
Felipe AF, Siong RLB, Uy HS (2009) Subconjunctival injection of bevacizumab for treatment of pterygium. Phillip J Ophthalmol 34(1):44–50
Khoshniat H, Jahadi Hosseini H, Nejabat M, Fatehi K, Mosallaei M (2009) Local injection of bevacizumab as an alternative method for management of recurrent pterygium. Iran Red Crescent Med J 11(3):306–311
Waisbourd M, Levinger E, Varssano D, Moisseiev E, Zayit-Soudri S, Barak A, Loewenstein A, Barequet I (2013) High-dose topical bevacizumab for corneal neovascularization. Pharmacology 92(5–6):310–314. doi:10.1159/000356407
Razeghinejad R, Banifatemi M, Hosseini H (2013) The effect of different doses of subconjunctival bevacizumab on the recurrence rate of excised primary pterygium. Bull Soc Belge Ophtalmol 322:13–20
Prabhasawat P, Barton K, Burkett G, Tseng SC (1997) Comparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excision. Ophthalmology 104(6):974–985
Acknowledgments
The authors wish to thank all the participants of this study and nursing staff of Imam Khomeini hospital for their valuable cooperation.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Motarjemizadeh, Q., Aidenloo, N.S. & Sepehri, S. A comparative study of different concentrations of topical bevacizumab on the recurrence rate of excised primary pterygium: a short-term follow-up study. Int Ophthalmol 36, 63–71 (2016). https://doi.org/10.1007/s10792-015-0076-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-015-0076-4