Abstract
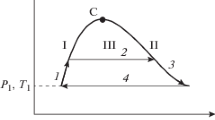
In 1960, E. H. Brown defined a set of characteristic curves (also known as ideal curves) of pure fluids, along which some thermodynamic properties match those of an ideal gas. These curves are used for testing the extrapolation behaviour of equations of state. This work is revisited, and an elegant representation of the first-order characteristic curves as level curves of a master function is proposed. It is shown that Brown’s postulate—that these curves are unique and dome-shaped in a double-logarithmic p, T representation—may fail for fluids exhibiting a density anomaly. A careful study of the Amagat curve (Joule inversion curve) generated from the IAPWS-95 reference equation of state for water reveals the existence of an additional branch.






Similar content being viewed by others
Notes
Brown furthermore defined a number of second-order characteristic curves, but we shall not deal with them here.
The derivations of the endpoint conditions and the terminal slopes are given in Appendix 2.
If metastable states are excluded; otherwise, the endpoint is on the liquid spinodal.
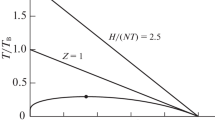
Equations of state matching the Thomas–Fermi asymptotics appear to be valid for materials at extremely high pressures as found in fusion plasmas and neutron stars [9]. Some researchers apply it to all states of aggregation, whereas others argue that this might be inappropriate for substances under terrestrial conditions where \(\lim _{p \rightarrow \infty } q_\mathrm{B}>\frac{5}{3}\) in nonplasma matter.
The supporting arguments of Brown [5] are not fully justified. Beyond the three laws of thermodynamics and the (physically reasonable) entropy condition \(\lim _{p \rightarrow \infty } (\partial S/\partial T)_p = 0\), he assumed the additional condition \(\lim _{p \rightarrow \infty } Z / p = v_\mathrm{min}(T)/RT >0\) at constant T, which is inappropriate in view of Thomas–Fermi theory.
This was claimed by Brown [5], based on the following—not convincing—argument: At a hypothetical point where two of the curves intersect, we must have \(c_{T} = Z/c_\kappa = 1\), hence all three curves intersect. Brown concluded without sufficient reason that Z should therefore attain a global minimum there, and that this is impossible.
The IAPWS-95 equation is officially valid up to 1273 K.
Abbreviations
- \(B_i\) :
-
ith virial coefficient
- \(C_p\) :
-
Isobaric heat capacity
- \(C_V\) :
-
Isochoric heat capacity
- \(c_X\) :
-
(Dimensionless) thermodynamic response function, \(X \in \{p, V, T, \kappa \}\) (Eqs. 14–15)
- G :
-
Gibbs energy
- \(\varvec{G}\) :
-
Hessian of \(G_\mathrm{m}(p, T)\)
- H :
-
Configurational enthalpy
- n :
-
Amount of substance
- p :
-
Pressure
- \(q_X\) :
-
Dimensionless logarithmic slope, \(X \in \{\mathrm{A, B, C}\}\) (Eqs. 16–18)
- R :
-
Universal gas constant
- S :
-
Configurational entropy
- T :
-
Temperature
- U :
-
Configurational internal energy
- V :
-
Volume
- Z :
-
Compression factor, \(Z = p V_\mathrm{m}/(R T)\)
- \(\alpha _p\) :
-
Isobaric thermal expansivity
- \(\kappa _T\) :
-
Isothermal compressibility
- \(\rho \) :
-
Molar density, \(\rho = V_\mathrm{m}^{-1}\)
- \(\mathrm{A}\) :
-
Amagat (Joule inversion) curve
- \(\mathrm{B}\) :
-
Boyle curve
- \(\mathrm{c}\) :
-
Critical property
- \(\mathrm{C}\) :
-
Charles (Joule–Thomson inversion) curve
- \(\mathrm{m}\) :
-
Molar property
- \(\mathrm{id}\) :
-
Ideal gas
- \(\mathrm{g}\) :
-
Vapour phase
- \(\mathrm{l}\) :
-
Liquid phase
References
W. Wagner, A. Pruß, J. Phys. Chem. Ref. Data 31, 387 (2002)
O. Kunz, R. Klimeck, W. Wagner, M. Jaeschke, The GERG-2004 Wide-Range Reference Equation of State for Natural Gases, vol. 15 of GERG (Groupe Européen de Recherches Gazières) Technical Monographs, VDI-Verlag, Düsseldorf (2007)
W. Wagner, J.R. Cooper, A. Dittmann, J. Kijima, H.-J. Kretzschmar, A. Kruse, R. Mareš, K. Oguchi, H. Sato, I. Stöcker, O. Šifner, Y. Takaishi, I. Tanishita, J. Trübenbach, T. Willkommen, J. Eng. Gas Turbines Power 122, 150 (2000)
W. Wagner, H.-J. Kretzschmar, International Steam Tables—Properties of Water and Steam Based on the Industrial Formulation IAPWS-IF97 (Springer, Berlin, 2008)
E .H. Brown, Bull. Int. Inst. Refrig. Paris Annex. 1960–1961, 169 (1960)
R. Span, W. Wagner, Int. J. Thermophys. 18, 1415 (1997)
U.K. Deiters, K.M. de Reuck, Pure Appl. Chem. 69, 1237 (1997). doi:10.1351/pac199769061237
J.P. Joule, W. Thomson, Phil. Mag. (Ser. 4) 4, 481 (1852)
J. Hama, K. Suito, J. Phys 8, 67 (1996)
P. Vinet, F. Ferrante, J.R. Smith, J.J. Rose, J. Phys. C 19, L467 (1986)
V. Holten, J. Sengers, M.A. Anisimov, J. Phys. Chem. Ref. Data 43, 043101:1 (2014)
J.S. Rowlinson, J. Chem. Phys. 19, 827 (1951)
K.M. Benjamin, A.J. Schultz, D.A. Kofke, J. Phys. Chem. C 111, 16021 (2007)
K.M. Benjamin, J.K. Singh, A.J. Schultz, D.A. Kofke, J. Phys. Chem. B 111, 11463 (2007)
A.J. Schultz, D.A. Kofke, A.H. Harvey, AIChE J. 61, 3029 (2015)
A. Schaber, Zum thermischen Verhalten fluider Stoffe—Eine systematische Untersuchung der charakteristischen Kurven des homogenen Zustandsgebiets, Ph.D. thesis, Technische Hochschule Karlsruhe, Germany (1965)
M .P. Vukalovich, M .S. Trakhtengerts, G .A. Spiridonov, Teploenergetica 14, 65 (1967) (in Russian)
G.S. Kell, G.E. McLaurin, E. Whalley, Proc. Royal Soc. Lond. 425, 49 (1989)
I.M. Abdulagatov, A.R. Bazaev, R.K. Gasanov, A.E. Ramazanova, J. Chem. Thermodyn. 28, 1037 (1996)
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1: Thermodynamic Derivatives
All derivatives are computed at constant amount of substance n.
isochoric tension coefficient:
derivatives appearing in Eq. 19:
Appendix 2: The Terminal Slopes of the Characteristic Curves at High Temperatures
Schaber presents expressions for the terminal slopes of Brown’s characteristic curves in his thesis [16], but does not give the proofs. For the readers’ convenience, these proofs are offered here.
For small pressures and large molar volumes the fluid can be described with a 3-term virial equation (cf. Eq. 20),
The molar volume as a function of pressure is then, neglecting higher-order terms,
1.1 (a) The Terminal Slope of the Amagat Curve
Applying the first one of the Amagat criteria in Eq. 2 to the virial equation yields
or
In the limit \(p \rightarrow 0\), \(V_\mathrm{m}\rightarrow \infty \) the second term can be neglected, and therefore \(B_2^\prime (T) = 0\) is the criterion for the endpoint of the Amagat curve, which corresponds to a maximum of the second virial coefficient.
Let \(T_\mathrm{A}\) denote the temperature of this endpoint, and \(\Delta T = T - T_\mathrm{A}\) and \(\Delta p = p\) the temperature and pressure deviations, respectively, from this point. Then, in the vicinity of \(T_\mathrm{A}\), \(B_2^\prime (T)\) can be approximated by
where \(B_2^{\prime \prime }(T_\mathrm{A})\) denotes the curvature of the second virial coefficient function at the Amagat endpoint. Substituting this into Eq. 30 and switching from molar volume to pressure yields
and, after some rearrangements,
In the limit \(\Delta p \rightarrow 0, T \rightarrow T_\mathrm{A}\) this reduces to
1.2 (b) The Terminal Slope of the Boyle Curve
Applying the criterion Eq. 5 to the virial equation gives
and hence
In the limit \(p \rightarrow 0\), this reduces to \(B_2 = 0\): At the endpoint of the Boyle curve, at the Boyle temperature \(T_\mathrm{B}\), the second virial coefficient vanishes.
Again using deviation variables, we can write the previous equation as
or
In the limit \(\Delta p \rightarrow 0, T \rightarrow T_\mathrm{B}\) this reduces to
1.3 (c) The Terminal Slope of the Charles Curve
For the derivation of this property, it is advantageous to start from the pressure-based virial equation of state,
The pressure-based virial coefficients are related to the volume-based ones by
Applying the appropriate criterion in Eq. 8 gives
or
The endpoint, at \(p \rightarrow 0\), is evidently characterized by \(B_2^\prime (T) - B_2(T)/T = 0\).
In order to obtain the terminal slope, we expand this criterion around the endpoint temperature \(T_\mathrm{C}\),
Then Eq. 36 becomes
In the limit \(\Delta p \rightarrow 0, T \rightarrow T_\mathrm{C}\), where the term in brackets vanishes, this yields the terminal slope,
Rights and permissions
About this article
Cite this article
Neumaier, A., Deiters, U.K. The Characteristic Curves of Water. Int J Thermophys 37, 96 (2016). https://doi.org/10.1007/s10765-016-2098-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-016-2098-1