Abstract
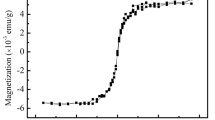
For the application of gadolinium oxide (Gd2O3) nanoparticles as terahertz contrast agents, their optical properties in a solvent were studied using terahertz time-domain spectroscopy. The power absorption and refractive index of the samples were measured with various concentrations of nanoparticles. The power absorption was extremely large, as much as three orders of magnitude higher than that of water, so that a few ppms of Gd2O3 nanoparticles were distinguished in terms of their power absorption capacity. The results show that the interaction between the terahertz electromagnetic waves and the Gd2O3 nanoparticles is strong enough to allow their exploitation as contrast agents for terahertz medical imaging.




Similar content being viewed by others
References
J.-H. Son, J. Appl. Phys. 105, 102033 (2009).
R.M. Woodward, V.P. Wallace, R.J. Pye, B.E. Cole, D.D. Arnone, E.H. Linfield, and M. Pepper, J. Invest. Dermatol. 120, 72–78 (2003).
A.J. Fitzgerald, V.P. Wallace, M. Jimenez-Linan, L. Bobrow, R.J. Pye, A.D. Purushotham, and D.D. Arnone, Radiology 239, 533–540 (2006).
P.C. Ashworth, E.Pickwell-MacPherson, E. Provenzano, S.E. Pinder, A.D. Purushotham, M. Pepper and V.P. Wallace, Opt. Express 17, 12444–12454 (2009).
J.-H. Lee, Y.-M. Huh, Y.-W. Jun, J.-W. Seo, J.-T. Jang, H.-T. Song, S. Kim, E.-J. Cho, H.-G. Yoon, J.-S. Suh, and J. Cheon, Nat. Med. 13, 95–99 (2007).
S.J. Oh, J. Kang, I. Maeng, J.-S. Suh, Y.-M. Huh, S. Haam, and J.-H. Son, Opt. Express 17, 3469–3475 (2009).
J. Lee, J. Yang, H. Ko, S.J. Oh, J. Kang, J.-H. Son, K. Lee, S.-W. Lee, H.-G. Yoon, J.-S. Suh, Y.-M. Huh, and S. Haam, Adv. Funct. Mater. 18, 258–264 (2008).
J. Kang, J. Yang, J. Lee, S.J. Oh, S. Moon, H.J. Lee, S.C. Lee, J.-H. Son, D. Kim, K. Lee, J.-S. Suh, Y.-M. Huh, and S. Haam, J. Mater. Chem., 19, 2902–2905 (2009).
J. Yang, J. Lee, J. Kang, S.J. Oh, H.-J. Ko, J.-H. Son, K. Lee, J.-S. Suh, Y.-M. Huh, and S. Haam, Adv. Mater. 21, 4339–4342 (2009).
J.-L. Bridot, A.-C. Faure, S. Laurent, C. Rivière, C. Billotey, B. Hiba, M. Janier, V. Josserand, J.-L. Coll, L.V. Elst, R. Muller, S. Roux, P. Perriat, and O. Tillement, J. Am. Chem. Soc. 129, 5076–5084 (2007).
J. Paek, C.H. Lee, J. Choi, S.-Y. Choi, A. Kim, J.W. Lee, and K. Lee, Cryst. Growth Des. 7, 1378–1380 (2007).
M. Born and E. Wolf, Principles of Optics, 7th ed. (Cambridge University Press, Cambridge, 1999)
L. Duvillaret, F. Garet, J.-F. Roux, and J.-L. Coutaz, IEEE J. Sel. Top. Quant. 2, 739 (1996).
D. S. Venables, A. Chiu, and C. A. Schmuttenmaer, J. Chem. Phys. 113, 3243 (2000).
P. Patnaik, Handbook of Inorganic Chemicals, (McGraw-Hill Professional, New York, 2002), pp. 305–306.
J.T. Kindt and C.A. Schmuttenmaer, J. Phys. Chem. 100, 10373 (1996).
S. J. Oh, J. Choi, I. Maeng, J. Y. Park, K. Lee, Y.-M. Huh, J.-S. Suh, S. Haam, and J.-H. Son, Opt. Express 19, 4009–4016 (2011).
Acknowledgements
This research was supported by the Basic Science Research Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (Nos. 2009–0083512, 2009–0054519, 2009–0076933, 2009–0093432, and 2009–0084187), by the grant of the Korean Health Technology R&D Project (No. A101954) funded by the Ministry for Health, Welfare & Family Affairs, Republic of Korea, and by Korea Small and Medium Business Administration in 2010 (00042838–1). DKL acknowledges the support of the Seoul Science Fellowship program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, DK., Kim, H., Kim, T. et al. Characteristics of Gadolinium Oxide Nanoparticles as Contrast Agents for Terahertz Imaging. J Infrared Milli Terahz Waves 32, 506–512 (2011). https://doi.org/10.1007/s10762-011-9776-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10762-011-9776-7