Abstract
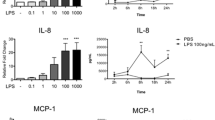
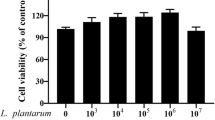
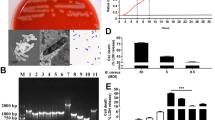
Intrauterine Escherichia coli infection after calving reduces fertility and causes major economic losses in the dairy industry. We investigated the protective effect of the probiotic Lactobacillus rhamnosus GR-1 on E. coli-induced cell damage and inflammation in primary bovine endometrial epithelial cells (BEECs). L. rhamnosus GR-1 reduced ultrastructure alterations and the percentage of BEECs apoptosis after E. coli challenge. Increased messenger RNA (mRNA) expression of immune response indicators, including pattern recognition receptors (toll-like receptor [TLR]2, TLR4, nucleotide-binding oligomerization domain [NOD]1, and NOD2), inflammasome proteins (NOD-like receptor family member pyrin domain-containing protein 3, apoptosis-associated speck-like protein, and caspase-1), TLR4 downstream adaptor molecules (myeloid differentiation antigen 88 [MyD88], toll-like receptor adaptor molecule 2 [TICAM2]), nuclear transcription factor kB (NF-kB), and the inflammatory cytokines tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, IL-8, IL-10, IL-18, and interferon (IFN)-β, was observed following E. coli challenge. However, these increases were attenuated by L. rhamnosus GR-1 pretreatment. Our data indicate that L. rhamnosus GR-1 ameliorates the E. coli-induced disruption of cellular ultrastructure, subsequently reducing the percentage of BEECs apoptosis and limiting inflammatory responses, partly via attenuation of MyD88-dependent and MyD88-independent pathway activation. Certain probiotics could potentially prevent postpartum uterine diseases in dairy cows, ultimately reducing the use of antibiotics.







Similar content being viewed by others
References
Sheldon, I.M., J. Cronin, L. Goetze, G. Donofrio, and H.J. Schuberth. 2009. Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle. Biology of Reproduction 81: 1025–1032.
Sheldon, I.M., E.J. Williams, A.N. Miller, D.M. Nash, and S. Herath. 2008. Uterine diseases in cattle after parturition. Veterinary Journal 176: 115–121.
Machado, V.S., M.L. Bicalho, R.V. Pereira, L.S. Caixeta, J.H. Bittar, G. Oikonomou, R.O. Gilbert, and R.C. Bicalho. 2012. The effect of intrauterine administration of mannose or bacteriophage on uterine health and fertility of dairy cows with special focus on Escherichia coli and Arcanobacterium pyogenes. Journal of Dairy Science 95: 3100–3109.
Williams, E.J., D.P. Fischer, D.E. Noakes, G.C. England, A. Rycroft, H. Dobson, and I.M. Sheldon. 2007. The relationship between uterine pathogen growth density and ovarian function in the postpartum dairy cow. Theriogenology 68: 549–559.
Dolejska, M., Z. Jurcickova, I. Literak, L. Pokludova, J. Bures, A. Hera, L. Kohoutova, J. Smola, and A. Cizek. 2011. IncN plasmids carrying bla CTX-M-1 in Escherichia coli isolates on a dairy farm. Veterinary Microbiology 149: 513–516.
Machado, V.S., M.L. Bicalho, E.B. Meira Junior, R. Rossi, B.L. Ribeiro, S. Lima, T. Santos, et al. 2014. Subcutaneous immunization with inactivated bacterial components and purified protein of Escherichia coli, Fusobacterium necrophorum and Trueperella pyogenes prevents puerperal metritis in Holstein dairy cows. PLoS One 9, e91734.
Kim, Y.G., T. Ohta, T. Takahashi, A. Kushiro, K. Nomoto, T. Yokokura, N. Okada, and H. Danbara. 2006. Probiotic Lactobacillus casei activates innate immunity via NF-kappaB and p38 MAP kinase signaling pathways. Microbes and Infection 8: 994–1005.
Karlsson, M., N. Scherbak, G. Reid, and J. Jass. 2012. Lactobacillus rhamnosus GR-1 enhances NF-kappaB activation in Escherichia coli-stimulated urinary bladder cells through TLR4. BMC Microbiology 12: 15.
Baldwin, A.S. 2012. Regulation of cell death and autophagy by IKK and NF-kappaB: critical mechanisms in immune function and cancer. Immunological Reviews 246: 327–345.
Reid, G., A.W. Bruce, N. Fraser, C. Heinemann, J. Owen, and B. Henning. 2001. Oral probiotics can resolve urogenital infections. FEMS Immunology and Medical Microbiology 30: 49–52.
Collado, M.C., E. Isolauri, S. Salminen, and Y. Sanz. 2009. The impact of probiotic on gut health. Current Drug Metabolism 10: 68–78.
Chan, R.C., A.W. Bruce, and G. Reid. 1984. Adherence of cervical, vaginal and distal urethral normal microbial flora to human uroepithelial cells and the inhibition of adherence of gram-negative uropathogens by competitive exclusion. Journal of Urology 131: 596–601.
Kim, S.O., H.I. Sheikh, S.D. Ha, A. Martins, and G. Reid. 2006. G-CSF-mediated inhibition of JNK is a key mechanism for Lactobacillus rhamnosus-induced suppression of TNF production in macrophages. Cellular Microbiology 8: 1958–1971.
Reid, G., and J. Burton. 2002. Use of Lactobacillus to prevent infection by pathogenic bacteria. Microbes and Infection 4: 319–324.
Yang, S., W. Li, J.R. Challis, G. Reid, S.O. Kim, and A.D. Bocking. 2014. Probiotic Lactobacillus rhamnosus GR-1 supernatant prevents lipopolysaccharide-induced preterm birth and reduces inflammation in pregnant CD-1 mice. American Journal of Obstetrics and Gynecology 211: 44.e41–44.e12.
Wu, Q., M.C. Liu, J. Yang, J.F. Wang, and Y.H. Zhu. 2016. Lactobacillus rhamnosus GR-1 ameliorates Escherichia coli-induced inflammation and cell damage via attenuation of ASC-independent NLRP3 inflammasome activation. Applied and Environmental Microbiology 82: 1173–1182.
Genís, S., À. Bach, F. Fàbregas, and A. Arís. 2016. Potential of lactic acid bacteria at regulating Escherichia coli infection and inflammation of bovine endometrium. Theriogenology 85: 625–637.
Ametaj, B.N., S. Iqbal, F. Selami, J.F. Odhiambo, Y. Wang, M.G. Ganzle, S.M. Dunn, and Q. Zebeli. 2014. Intravaginal administration of lactic acid bacteria modulated the incidence of purulent vaginal discharges, plasma haptoglobin concentrations, and milk production in dairy cows. Research in Veterinary Science 96: 365–370.
Deng, Q., J.F. Odhiambo, U. Farooq, T. Lam, S.M. Dunn, and B.N. Ametaj. 2015. Intravaginal lactic acid bacteria modulated local and systemic immune responses and lowered the incidence of uterine infections in periparturient dairy cows. PLoS One 10, e0124167.
MacKintosh, S.B., H.J. Schuberth, L.L. Healy, and I.M. Sheldon. 2013. Polarised bovine endometrial epithelial cells vectorially secrete prostaglandins and chemotactic factors under physiological and pathological conditions. Reproduction 145: 57–72.
Turner, M.L., J.G. Cronin, G.D. Healey, and I.M. Sheldon. 2014. Epithelial and stromal cells of bovine endometrium have roles in innate immunity and initiate inflammatory responses to bacterial lipopeptides in vitro via Toll-like receptors TLR2, TLR1, and TLR6. Endocrinology 155: 1453–1465.
King, A.E., A.W. Horne, S. Hombach-Klonisch, J.I. Mason, and H.O. Critchley. 2009. Differential expression and regulation of nuclear oligomerization domain proteins NOD1 and NOD2 in human endometrium: a potential role in innate immune protection and menstruation. Molecular Human Reproduction 15: 311–319.
Roshangar, L., S. Abdollahifard, A. Majdi, A. Zarrintan, A. Ghasemzade, L. Farzadi, S.S. Rad, and J.S. Rad. 2013. Study of ultrastructure and apoptosis in the endometrium of women with or without endometriosis. Iranian Journal of Reproductive Medicine 11: 399–404.
Cronin, J.G., M.L. Turner, L. Goetze, C.E. Bryant, and I.M. Sheldon. 2012. Toll-like receptor 4 and MYD88-dependent signaling mechanisms of the innate immune system are essential for the response to lipopolysaccharide by epithelial and stromal cells of the bovine endometrium. Biology of Reproduction 86: 51.
Fu, Y., B. Liu, X. Feng, Z. Liu, D. Liang, F. Li, D. Li, et al. 2013. Lipopolysaccharide increases Toll-like receptor 4 and downstream Toll-like receptor signaling molecules expression in bovine endometrial epithelial cells. Veterinary Immunology and Immunopathology 151: 20–27.
Sheldon, I.M., A.N. Rycroft, B. Dogan, M. Craven, J.J. Bromfield, A. Chandler, M.H. Roberts, S.B. Price, R.O. Gilbert, and K.W. Simpson. 2010. Specific strains of Escherichia coli are pathogenic for the endometrium of cattle and cause pelvic inflammatory disease in cattle and mice. PLoS One 5, e9192.
Galvão, K.N., N.R. Santos, J.S. Galvão, and R.O. Gilbert. 2011. Association between endometritis and endometrial cytokine expression in postpartum Holstein cows. Theriogenology 76: 290–299.
Turner, M.L., G.D. Healey, and I.M. Sheldon. 2012. Immunity and inflammation in the uterus. Reproduction in Domestic Animals 47(Suppl 4): 402–409.
Fortier, M.A., L.A. Guilbault, and F. Grasso. 1988. Specific properties of epithelial and stromal cells from the endometrium of cows. Journal of Reproduction and Fertility 83: 239–248.
Horne, A.W., E.N. Lalani, R.A. Margara, T.A. Ryder, M.A. Mobberley, and J.O. White. 2005. The expression pattern of MUC1 glycoforms and other biomarkers of endometrial receptivity in fertile and infertile women. Molecular Reproduction and Development 72: 216–229.
Liu, D., Y. Yang, Q. Liu, and J. Wang. 2011. Inhibition of autophagy by 3-MA potentiates cisplatin-induced apoptosis in esophageal squamous cell carcinoma cells. Medical Oncology 28: 105–111.
Ma, J.L., Y.H. Zhu, L. Zhang, Z.Y. Zhuge, P.Q. Liu, X.D. Yan, H.S. Gao, and J.F. Wang. 2011. Serum concentration and mRNA expression in milk somatic cells of toll-like receptor 2, toll-like receptor 4, and cytokines in dairy cows following intramammary inoculation with Escherichia coli. Journal of Dairy Science 94: 5903–5912.
Földi, J., M. Kulcsár, A. Pecsi, B. Huyghe, C. de Sa, J.A. Lohuis, P. Cox, and G. Huszenicza. 2006. Bacterial complications of postpartum uterine involution in cattle. Animal Reproduction Science 96: 265–281.
Long, E., A.V. Capuco, D.L. Wood, T. Sonstegard, G. Tomita, M.J. Paape, and X. Zhao. 2001. Escherichia coli induces apoptosis and proliferation of mammary cells. Cell Death and Differentiation 8: 808–816.
Okazaki, M., T. Matsuyama, T. Kohno, H. Shindo, T. Koji, Y. Morimoto, and T. Ishimaru. 2005. Induction of epithelial cell apoptosis in the uterus by a mouse uterine ischemia-reperfusion model: possible involvement of tumor necrosis factor-alpha. Biology of Reproduction 72: 1282–1288.
Yan, F., and D.B. Polk. 2002. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. Journal of Biological Chemistry 277: 50959–50965.
Lappas, M. 2013. NOD1 and NOD2 regulate proinflammatory and prolabor mediators in human fetal membranes and myometrium via nuclear factor-kappa B. Biology of Reproduction 89: 14.
Zhang, W., Y.H. Zhu, J.C. Yang, G.Y. Yang, D. Zhou, and J.F. Wang. 2015. A selected Lactobacillus rhamnosus strain promotes EGFR-independent Akt activation in an enterotoxigenic Escherichia coli K88-infected IPEC-J2 cell model. PLoS One 10, e0125717.
Herath, S., D.P. Fischer, D. Werling, E.J. Williams, S.T. Lilly, H. Dobson, C.E. Bryant, and I.M. Sheldon. 2006. Expression and function of Toll-like receptor 4 in the endometrial cells of the uterus. Endocrinology 147: 562–570.
Young, S.L., T.D. Lyddon, R.L. Jorgenson, and M.L. Misfeldt. 2004. Expression of Toll-like receptors in human endometrial epithelial cells and cell lines. American Journal of Reproductive Immunology 52: 67–73.
Borges, A.M., G.D. Healey, and I.M. Sheldon. 2012. Explants of intact endometrium to model bovine innate immunity and inflammation ex vivo. American Journal of Reproductive Immunology 67: 526–539.
Wagner, R.D., and S.J. Johnson. 2012. Probiotic lactobacillus and estrogen effects on vaginal epithelial gene expression responses to Candida albicans. Journal of Biomedical Science 19: 58.
Fitzgerald, K.A., D.C. Rowe, B.J. Barnes, D.R. Caffrey, A. Visintin, E. Latz, B. Monks, P.M. Pitha, and D.T. Golenbock. 2003. LPS-TLR4 signaling to IRF-3/7 and NF-kappaB involves the toll adapters TRAM and TRIF. Journal of Experimental Medicine 198: 1043–1055.
Van Gorp, H., A. Kuchmiy, F. Van Hauwermeiren, and M. Lamkanfi. 2014. NOD-like receptors interfacing the immune and reproductive systems. FEBS Journal 281: 4568–4582.
Burberry, A., M.Y. Zeng, L. Ding, I. Wicks, N. Inohara, S.J. Morrison, and G. Núñez. 2014. Infection mobilizes hematopoietic stem cells through cooperative NOD-like receptor and Toll-like receptor signaling. Cell Host & Microbe 15: 779–791.
Latz, E. 2010. The inflammasomes: mechanisms of activation and function. Current Opinion in Immunology 22: 28–33.
Man, S.M., and T.D. Kanneganti. 2015. Regulation of inflammasome activation. Immunological Reviews 265: 6–21.
Acknowledgments
This work was supported by grants from the Program for the Beijing Dairy Industry Innovation Team and the National Natural Science Foundation of China (Project Nos. 31372493).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, M., Wu, Q., Wang, M. et al. Lactobacillus rhamnosus GR-1 Limits Escherichia coli-Induced Inflammatory Responses via Attenuating MyD88-Dependent and MyD88-Independent Pathway Activation in Bovine Endometrial Epithelial Cells. Inflammation 39, 1483–1494 (2016). https://doi.org/10.1007/s10753-016-0382-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-016-0382-7