Abstract
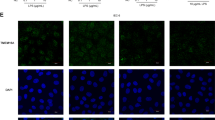
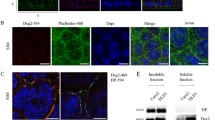
The effect of oxidative stress on barrier integrity and localization of transmembrane serine proteinase 2 (TMPRSS2) were studied using porcine epithelial IPEC-J2 cells on membrane inserts. Increased paracellular permeability of FITC-dextran 4 kDa (fluorescence intensity 43,508 ± 2,391 versus 3,550 ± 759) and that of gentamicin (3.41 ± 0.06 % increase to controls) were measured parallel with the reduced transepithelial electrical resistance (23.3 ± 4.06 % decrease) of cell layers 6 h after 1 h 1 mM H2O2 treatment. The immunohistochemical localization of adherens junctional β-catenin was not affected by reactive oxygen species (ROS) up to 4 mM H2O2. Peroxide-triggered enhanced paracellular permeability of IPEC-J2 cell layer was accompanied by predominantly cytoplasmic occurrence of TMPRSS2 embedded in cell membrane under physiological conditions. These results support that ROS can influence paracellular gate opening via multifaceted mode of action without involvement of β-catenin redistribution in adherens junction. Altered distribution pattern of TMPRSS2 and relocalized transmembrane serine protease activity may contribute to weakening of epithelial barrier integrity under acute oxidative stress.




Similar content being viewed by others
REFERENCES
Arnett, H.A., and J.L. Viney. 2010. Gatekeepers of intestinal inflammation. Inflammation Research 59(1): 1–14.
Hartman, K.G., J.D. Bortner, G.W. Falk, et al. 2013. Modeling inflammation and oxidative stress in gastrointestinal disease development using novel organotypic culture systems. Stem Cell Research & Therapy 4(Suppl 1): S5. doi:10.1186/scrt366.
Daugherty, R.L., and C.J. Gottardi. 2007. Phospho-regulation of β-catenin adhesion and signaling functions. APS Physiology 22: 303–309.
Huber, A.H., D.B. Stewart, D.V. Laurents, et al. 2001. The cadherin cytoplasmic domain is unstructured in the absence of beta-catenin. A possible mechanism for regulating cadherin turnover. Journal of Biological Chemistry 276: 12301–12309.
Drees, F., S. Pokutta, S. Yamada, et al. 2005. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell 123: 903–915.
Yamada, S., S. Pokutta, F. Drees, et al. 2005. Deconstructing the cadherin-catenin-actin complex. Cell 123: 889–901.
Guntaka, S.R., G. Samak, A. Seth, et al. 2011. Epidermal growth factor protects the apical junctional complexes from hydrogen peroxide in bile duct epithelium. Laboratory Investigation 91(9): 1396–1409.
Rao, R.K., S. Basuroy, V.U. Rao, et al. 2002. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-β-catenin complexes from the cytoskeleton by oxidative stress. Biochemical Journal 368(2): 471–481.
Seth, A., P. Sheth, B.C. Elias, et al. 2007. Protein phosphatases 2A and 1 interact with occludin and negatively regulate the assembly of tight junctions in the CaCo-2 cell monolayer. Journal of Biological Chemistry 282(15): 11487–11498.
Meyer, T.N., C. Schwesinger, J. Ye, et al. 2001. Reassembly of the tight junction after oxidative stress depends on tyrosine kinase activity. Journal of Biological Chemistry 276(25): 22048–22055.
Covacci, V., A. Torsello, P. Palozza, et al. 2001. DNA oxidative damage during differentiation of HL-60 human promyelocytic leukemia cells. Chemical Research Toxicology 14(11): 1492–1497.
Kojima, T., T. Norose, K. Tsuchiya, et al. 2010. Mouse 3T3-L1 cells acquire resistance against oxidative stress as the adipocytes differentiate via the transcription factor FoxO. Apoptosis 15(1): 83–93.
Schneider, L., S. Giordano, B.R. Zelickson, et al. 2001. Differentiation of SH-SY5Y cells to a neuronal phenotype changes cellular bioenergetics and the response to oxidative stress. Free Radical Biology & Medicine 51(11): 2007–2017.
Rao, R.K., R.D. Baker, S.S. Baker, et al. 1997. Oxidant-induced disruption of intestinal epithelial barrier function: role of protein tyrosine phosphorylation. AJP- Gastrointestinal Liver Physiology 273: G812–G823.
Forsyth, C.B., A. Banan, A. Farhadi, et al. 2007. Regulation of oxidant-induced intestinal permeability by metalloprotease-dependent epidermal growth factor receptor signaling. Journal of Pharmacology and Experimental Therapeutics 321(1): 84–97.
Theiss, A.L., R.D. Idell, S. Srinivasan, et al. 2007. Prohibitin protects against oxidative stress in intestinal epithelial cells. FASEB Journal 21(1): 197–206.
Temmesfeld-Wollbrück, B., B. Brell, C. zu Dohna, et al. 2009. Adrenomedullin reduces intestinal epithelial permeability in vivo and in vitro. AJP-Gastrointestinal Liver Physiology 297(1): G43–G51.
Brosnahan, A., and D.R. Brown. 2012. Porcine epithelial cells in microbiological investigations. Veterinary Microbiology 156(3–4): 229–237.
Schierack, P., M. Nordhoff, M. Pollmann, et al. 2006. Characterization of a porcine intestinal epithelial cell line for in vitro studies of microbial pathogenesis in swine. Histochemistry and Cell Biology 125: 293–305.
Madara, J.L. 1989. Loosening tight junctions. Lessons from the intestine. Journal of Clinical Investigation 83: 1089–1094.
Miyauchi, E., H. Morita, and S. Tanabe. 2009. Lactobacillus rhamnosus alleviates intestinal barrier dysfunction in part by increasing expression of zonula occludens-1 and myosin light-chain kinase in vivo. Journal of Diary Science 92: 2400–2408.
Qin, H., Z. Zhang, X. Hang, and Y.L. Jiang. 2009. L. plantarum prevents enteroinvasive Escherichia coli-induced tight junction proteins changes in intestinal epithelial cells. BMC Microbiology 9: 63.
Mennigen, R.K., E. Rijcken Nolte, et al. 2009. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. AJP-Gastrointestinal Liver Physiology 296: G1140–G1149.
Isoherranen, N., E. Lavy, and S. Soback. 2000. Pharmacokinetics of gentamicin C1, C1a and C2 in beagles after a single intravenous dose. Antimicrobial Agents and Chemotherapy 44(6): 1443–1447.
Soltes, L. 1999. Aminoglycoside antibiotics—two decades of their HPLC bioanalysis. Biomedical Chromatography 13: 3–10.
Lacy, J.E., R.T. Parfitt, and M.G. Rowan. 1988. The effects of column packing material and inorganic cations on the separation of fluorescent O-phthalaldehyde derivatives of gentamicin by high-performance liquid chromatography. International Journal of Pharmaceutics 43(1–2): 111–117.
Stead, D.A., and R.M.E. Richards. 1996. Sensitive fluorimetric determination of gentamicin sulfate in biological matrices using solid-phase extraction, pre-column derivatization with 9-fluorenylmethyl chloroformate and reversed-phase high-performance liquid chromatography. Journal of Chromatography B 675(2): 295–302.
Chen, C.-J., B.-Y. Wu, P.-I. Tsao, et al. 2011. Increased matriptase zymogen activation in inflammatory skin disorders. AJP Cell Physiology 300(3): 406–415.
Buzza, M.S., S. Netzel-Arnett, T. Shea-Donohue, et al. 2010. Membrane-anchored serine protease matriptase regulates epithelial barrier formation and permeability in the intestine. Proceedings of the National Academy of Sciences 107(9): 4200–4205.
Hammami, M., E. Rühmann, E. Maurer, et al. 2012. New 3-amidinophenylalanine-derived inhibitors of matriptase. Medicinal Chemistry Communications 3: 807–813.
Tremblay, E., J. Auclair, E. Delvin, et al. 2006. Gene expression profiles of normal proliferating and differentiating human intestinal epithelial cells: a comparison with the Caco-2 cell model. Journal of Cell Biochemistry 99(4): 1175–1186.
Langerholc, T., P.A. Maragkoudakis, J. Wollgast, et al. 2011. Novel and established intestinal cell line models—an indispensable tool in food science and nutrition. Trends in Food Science & Technology 22(1): S11–S20.
Paszti-Gere, E., G. Matis, O. Farkas, et al. 2014. The effects of intestinal LPS exposure on inflammatory responses in a porcine enterohepatic co-culture system. Inflammation 37(1): 247–260.
Cencic, A., and T. Langerholc. 2010. Functional cell models of the gut and their applications in food microbiology—a review. International Journal of Food Microbiology 141: 4–14.
Harris, M.L., H.J. Schiller, P.M. Reilly, et al. 1992. Free radicals and other reactive oxygen metabolites in inflammatory bowel disease: cause, consequence or epiphenomenon? Pharmacology & Therapeutics 53: 375–408.
Blau, S., A. Rubinstein, P. Bass, et al. 1999. Differences in the reducing power along the rat GI tract: lower antioxidant capacity of the colon. Molecular Cellular Biochemistry 194: 185–191.
Giandomenico, A.R., G.E. Cerniglia, J.E. Biaglow, et al. 1997. The importance of sodium pyruvate in assessing damage produced by hydrogen peroxide. Free Radical Biology & Medicine 23: 426–434.
Chapman, K.E., C.M. Waters, and W.M. Miller. 2002. Continuous exposure of airway epithelial cells to hydrogen peroxide: protection by KGF. Journal of Cell Physiology 192(1): 71–80.
Lee, B.C., T.H. Lee, S. Avraham, et al. 2004. Involvement of the chemokine receptor CXCR4 and its ligand stromal cell-derived factor 1alpha in breast cancer cell migration through human brain microvascular endothelial cells. Molecular Cancer Research 2(1): 327–338.
González-Mariscal, L., A. Betanzos, P. Nava, et al. 2003. Tight junction proteins. Progress in Biophysics and Molecular Biology 81: 1–44.
Oliveira, S.S., and J.A. Morgado-Díaz. 2007. Claudins: multifunctional players in epithelial tight junctions and their role in cancer. Cellular and Molecular Life Sciences 64(1): 17–28.
Paszti-Gere, E., E. Csibrik-Nemeth, K. Szeker, et al. 2012. Acute oxidative stress affects IL-8 and TNF-α expression in IPEC-J2 porcine epithelial cells. Inflammation 35(3): 994–1004.
Lucas, J.M., L. True, S. Hawley, et al. 2008. The androgen-regulated type II serine protease TMPRSS2 is differentially expressed and mislocalized in prostate adenocarcinoma. Journal of Pathology 215: 118–125.
Paszti-Gere, E., K. Szeker, E. Csibrik-Nemeth, et al. 2012. Metabolites of Lactobacillus plantarum 2142 prevent oxidative stress-induced overexpression of proinflammatory cytokines in IPEC-J2 cell line. Inflammation 35(4): 1487–1499.
ACKNOWLEDGMENTS
The research was supported by the Hungarian Scientific Research Fund (grant numbers are 100701 and 109865). This research was supported by the European Union and the State of Hungary, co-financed by the European Social Fund in the framework of TÁMOP 4.2.4. A/2-11-1-2012-0001 ‘National Excellence Program’ and by Research Faculty Grant 2014 of the Szent István University, Faculty of Veterinary Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paszti-Gere, E., Barna, R.F., Kovago, C. et al. Changes in the Distribution of Type II Transmembrane Serine Protease, TMPRSS2 and in Paracellular Permeability in IPEC-J2 Cells Exposed to Oxidative Stress. Inflammation 38, 775–783 (2015). https://doi.org/10.1007/s10753-014-9988-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-014-9988-9