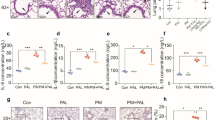
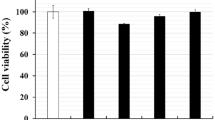
Abstract
Mastitis comprises an inflammation of the mammary gland, which is almost always linked with bacterial infection. The treatment of mastitis concerns antimicrobial substances, but not very successful. On the other hand, anti-inflammatory therapy with Chinese traditional medicine becomes an effective way for treating mastitis. Magnolol is a polyphenolic binaphthalene compound extracted from the stem bark of Magnolia sp., which has been shown to exert a potential for anti-inflammatory activity. The purpose of this study was to investigate the protective effects of magnolol on inflammation in lipopolysaccharide (LPS)-induced mastitis mouse model in vivo and the mechanism of this protective effects in LPS-stimulated mouse mammary epithelial cells (MMECs) in vitro. The damage of tissues was determined by histopathology and myeloperoxidase (MPO) assay. The expression of pro-inflammatory cytokines was determined by enzyme-linked immunosorbent assay (ELISA). Nuclear factor-kappa B (NF-κB), inhibitory kappa B (IκBα) protein, p38, extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and Toll-like receptor 4 (TLR4) were determined by Western blot. The results showed that magnolol significantly inhibit the LPS-induced TNF-α, IL-6, and IL-1β production both in vivo and vitro. Magnolol declined the phosphorylation of IκBα, p65, p38, ERK, and JNK in LPS-stimulated MMECs. Furthermore, magnolol inhibited the expression of TLR4 in LPS-stimulated MMECs. In vivo study, it was also observed that magnolol attenuated the damage of mastitis tissues in the mouse models. These findings demonstrated that magnolol attenuate LPS-stimulated inflammatory response by suppressing TLR4/NF-κB/mitogen-activated protein kinase (MAPK) signaling system. Thereby, magnolol may be a therapeutic agent against mastitis.








Similar content being viewed by others
REFERENCES
Watts, J.L. 1988. Etiological agents of bovine mastitis. Veterinary Microbiology 16: 41–66.
Burvenich, C., V. Van Merris, J. Mehrzad, A. Diez-Fraile, and L. Duchateau. 2003. Severity of E. coli mastitis is mainly determined by cow factors. Veterinary Research 34: 521–564.
Vangroenweghe, F., P. Rainard, M. Paape, L. Duchateau, and C. Burvenich. 2004. Increase of Escherichia coli inoculum doses induces faster innate immune response in primiparous cows. Journal of Dairy Science 87: 4132–4144.
Li, Y., G.V. Limmon, F. Imani, and C. Teng. 2009. Induction of lactoferrin gene expression by innate immune stimuli in mouse mammary epithelial HC-11 cells. Biochimie 91: 58–67.
Strandberg, Y., C. Gray, T. Vuocolo, L. Donaldson, M. Broadway, and R. Tellam. 2005. Lipopolysaccharide and lipoteichoic acid induce different innate immune responses in bovine mammary epithelial cells. Cytokine 31: 72–86.
Reuven, E.M., A. Fink, and Y. Shai. 2014. Regulation of innate immune responses by transmembrane interactions: lessons from the TLR family. Biochimica et Biophysica Acta 1838(6): 1586–1593.
Ariyadi, B., N. Isobe, and Y. Yoshimura. 2014. Toll-like receptor signaling for the induction of mucin expression by lipopolysaccharide in the hen vagina. Poultry Science 93: 673–679.
Kyriakis, J.M., and J. Avruch. 2001. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiological Reviews 81: 807–869.
Lee, J.C., S. Kassis, S. Kumar, A. Badger, and J.L. Adams. 1999. p38 mitogen-activated protein kinase inhibitors-mechanisms and therapeutic potentials. Pharmacology and Therapeutics 82: 389–397.
Riollet, C., Rainard, P., Poutrel B. 2002 Cells and cytokines in inflammatory secretions of bovine mammary gland. In Biology of the Mammary Gland, 247-258. Springer.
Watanabe, K., H. Watanabe, Y. Goto, M. Yamaguchi, N. Yamamoto, and K. Hagino. 1983. Pharmacological properties of magnolol and honokiol extracted from Magnolia officinalis: central depressant effects. Planta Medica 49: 103–108.
Blakey, D.H., J.M. Bayley, and K.C. Huang. 1993. Suitability of human chromosome-specific DNA libraries for mutagenicity studies in Macaca fascicularis. Mutagenesis 8: 189–192.
Son, H.J., H.J. Lee, H.S. Yun-Choi, and J.H. Ryu. 2000. Inhibitors of nitric oxide synthesis and TNF-alpha expression from Magnolia obovata in activated macrophages. Planta Medica 66: 469–471.
Park, J., J. Lee, E. Jung, Y. Park, K. Kim, B. Park, K. Jung, E. Park, J. Kim, and D. Park. 2004. In vitro antibacterial and anti-inflammatory effects of honokiol and magnolol against Propionibacterium sp. European Journal of Pharmacology 496: 189–195.
Fu, Y., B. Liu, N. Zhang, Z. Liu, D. Liang, F. Li, Y. Cao, X. Feng, X. Zhang, and Z. Yang. 2013. Magnolol inhibits lipopolysaccharide-induced inflammatory response by interfering with TLR4 mediated NF-kappaB and MAPKs signaling pathways. Journal of Ethnopharmacology 145: 193–199.
Li, D., Y. Fu, W. Zhang, G. Su, B. Liu, M. Guo, F. Li, D. Liang, Z. Liu, X. Zhang, et al. 2013. Salidroside attenuates inflammatory responses by suppressing nuclear factor-kappaB and mitogen activated protein kinases activation in lipopolysaccharide-induced mastitis in mice. Inflammation Research 62: 9–15.
Smalley, M.J. 2010. Isolation, culture and analysis of mouse mammary epithelial cells. Methods in Molecular Biology 633: 139–170.
Li, D., N. Zhang, Y. Cao, W. Zhang, G. Su, Y. Sun, Z. Liu, F. Li, D. Liang, B. Liu, et al. 2013. Emodin ameliorates lipopolysaccharide-induced mastitis in mice by inhibiting activation of NF-κB and MAPKs signal pathways. European Journal of Pharmacology 705: 79–85.
Li, Q., and I.M. Verma. 2002. NF-kappaB regulation in the immune system. Nature Reviews Immunology 2: 725–734.
Jung, W.K., D.Y. Lee, C. Park, Y.H. Choi, I. Choi, S.G. Park, S.K. Seo, S.W. Lee, S.S. Yea, S.C. Ahn, et al. 2010. Cilostazol is anti-inflammatory in BV2 microglial cells by inactivating nuclear factor-kappaB and inhibiting mitogen-activated protein kinases. British Journal of Pharmacology 159: 1274–1285.
Zhao, X., and P. Lacasse. 2008. Mammary tissue damage during bovine mastitis: causes and control. Journal of Animal Science 86: 57–65.
Viguier, C., S. Arora, N. Gilmartin, K. Welbeck, and R. O’Kennedy. 2009. Mastitis detection: current trends and future perspectives. Trends in Biotechnology 27: 486–493.
Babra, C., J.G. Tiwari, G. Pier, T.H. Thein, R. Sunagar, S. Sundareshan, S. Isloor, N.R. Hegde, S. de Wet, and M. Deighton. 2013. The persistence of biofilm-associated antibiotic resistance of Staphylococcus aureus isolated from clinical bovine mastitis cases in Australia. Folia Microbiologica 58: 469–474.
Senegas, A., O. Villard, A. Neuville, L. Marcellin, A.W. Pfaff, T. Steinmetz, M. Mousli, J.P. Klein, and E. Candolfi. 2009. Toxoplasma gondii-induced foetal resorption in mice involves interferon-gamma-induced apoptosis and spiral artery dilation at the maternofoetal interface. International Journal for Parasitology 39: 481–487.
Takahashi, K., H. Harada, S.W. Schaffer, and J. Azuma. 1992. Effect of taurine on intracellular calcium dynamics of cultured myocardial cells during the calcium paradox. Advances in Experimental Medicine and Biology 315: 153–161.
Li, F., D. Liang, Z. Yang, T. Wang, W. Wang, X. Song, M. Guo, E. Zhou, D. Li, and Y. Cao. 2013. Astragalin suppresses inflammatory responses via down-regulation of NF-κB signaling pathway in lipopolysaccharide-induced mastitis in a murine model. International Immunopharmacology 17: 478–482.
Song, X., Zhang, W., Wang, T., Jiang, H., Zhang, Z., Fu, Y., Yang, Z., Cao, Y., Zhang, N. 2014. Geniposide plays an anti-inflammatory role via regulating TLR4 and downstream signaling pathways in lipopolysaccharide-induced mastitis in mice. Inflammation 1-11.
Fried, L.E., and J.L. Arbiser. 2009. Honokiol, a multifunctional antiangiogenic and antitumor agent. Antioxidants and Redox Signaling 11: 1139–1148.
Zheng, J., A.D. Watson, and D.E. Kerr. 2006. Genome-wide expression analysis of lipopolysaccharide-induced mastitis in a mouse model. Infection and Immunity 74: 1907–1915.
Krawisz, J., P. Sharon, and W. Stenson. 1984. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Gastroenterology 87: 1344–1350.
Higashimoto, T., A. Panopoulos, C.L. Hsieh, and E. Zandi. 2006. TNFalpha induces chromosomal abnormalities independent of ROS through IKK, JNK, p38 and caspase pathways. Cytokine 34: 39–50.
Yoon, W.J., J.Y. Moon, J.Y. Kang, G.O. Kim, N.H. Lee, and C.G. Hyun. 2010. Neolitsea sericea essential oil attenuates LPS-induced inflammation in RAW 264.7 macrophages by suppressing NF-kappaB and MAPK activation. Natural Product Communications 5: 1311–1316.
Ho, A.W., C.K. Wong, and C.W. Lam. 2008. Tumor necrosis factor-alpha up-regulates the expression of CCL2 and adhesion molecules of human proximal tubular epithelial cells through MAPK signaling pathways. Immunobiology 213: 533–544.
Shalaby, M.R., B.B. Aggarwal, E. Rinderknecht, L.P. Svedersky, B.S. Finkle, and M.A. Palladino Jr. 1985. Activation of human polymorphonuclear neutrophil functions by interferon-gamma and tumor necrosis factors. Journal of Immunology 135: 2069–2073.
Oh, Y.C., W.K. Cho, Y.H. Jeong, G.Y. Im, A. Kim, Y.H. Hwang, T. Kim, K.H. Song, and J.Y. Ma. 2012. A novel herbal medicine KIOM-MA exerts an anti-inflammatory effect in LPS-stimulated RAW 264.7 macrophage cells. Evidence-Based Complementary and Alternative Medicine 2012: 462383.
Hayden, M.S., and S. Ghosh. 2012. NF-kappaB, the first quarter-century: remarkable progress and outstanding questions. Genes and Development 26: 203–234.
Vallabhapurapu, S., and M. Karin. 2009. Regulation and function of NF-kappaB transcription factors in the immune system. Annual Review of Immunology 27: 693–733.
Liang, C.J., C.W. Lee, H.C. Sung, Y.H. Chen, Y.C. Chiang, H.Y. Hsu, Y.C. Tseng, C.Y. Li, S.H. Wang, and Y.L. Chen. 2014. Ganoderma lucidum polysaccharides reduce lipopolysaccharide-induced interleukin-1 beta expression in cultured smooth muscle cells and in thoracic aortas in mice. Evidence-based Complementary and Alternative Medicine 2014: 305149.
Remppis, A., F. Bea, H.J. Greten, A. Buttler, H. Wang, Q. Zhou, M.R. Preusch, R. Enk, R. Ehehalt, H. Katus, and E. Blessing. 2010. Rhizoma Coptidis inhibits LPS-induced MCP-1/CCL2 production in murine macrophages via an AP-1 and NFkappaB-dependent pathway. Mediators of Inflammation 2010: 194896.
Kaminska, B. 2005. MAPK signalling pathways as molecular targets for anti-inflammatory therapy-from molecular mechanisms to therapeutic benefits. Biochimica et Biophysica Acta 1754: 253–262.
Medzhitov, R., and J.C. Kagan. 2006. Phosphoinositide-mediated adaptor recruitment controls toll-like receptor signaling. Cell 125: 943–955.
ACKNOWLEDGMENTS
This work was supported by a grant from the National Natural Science Foundation of China (Nos. 31272622, 31201925), the Research Fund for the Doctoral Program of Higher Education of China (Nos. 20110061130010, 20120061120098), and Jilin Province Science Foundation for Youths (No. 20130522087JH).
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors’ Contributions
Naisheng Zhang and Zhengtao Yang conceived and designed the paper. Wei Wang, Xiaojing Song, and Tiancheng Wang executed the experiment and analyzed the samples. Dejie Liang and Yongguo Cao analyzed the data. All authors interpreted the data, critically revised the manuscript for important intellectual contents, and approved the final version.
Rights and permissions
About this article
Cite this article
Wei, W., Dejie, L., Xiaojing, S. et al. Magnolol Inhibits the Inflammatory Response in Mouse Mammary Epithelial Cells and a Mouse Mastitis Model. Inflammation 38, 16–26 (2015). https://doi.org/10.1007/s10753-014-0003-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-014-0003-2