Abstract
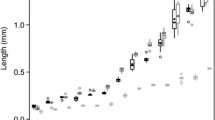
Understanding species interactions and how they change in the presence of a predator or competitor is a fundamental goal for ecologists. We tested such interactions in an intertidal soft sediment pool system where both the sand goby Favonigobius lentiginosus and post-settlement whiting Sillago spp. consume meiofaunal prey, but F. lentiginosus also consumes Sillago spp. We quantified changes in fish gut content volumes and composition (i.e. meiofaunal group diversity and abundances) in response to the presence of a predator/prey and to different fish densities (two, four or six total individuals) in experimental aquaria. We found no significant density-dependent effects on either the predator or prey species, likely due to meiofaunal prey oversupply; however, the diet composition of the prey species Sillago spp. changed significantly in the presence of their potential predator. We conclude that lower consumption of meiofaunal amphipods in the presence of gobies suggests that whiting perhaps maintain their gut fullness by preferentially targeting larger amphipods.




Similar content being viewed by others
References
Anderson, M. J. & T. J. Willis, 2003. Canonical analysis of principal coordinates: a useful method of constrained ordination for ecology. Ecology 84(2): 511–525.
Anderson, M. J., R. N. Gorley & K. R. Clarke, 2008. PERMANOVA+ for Primer: guide to software and statistical methods. PRIMER-E Ltd, Plymouth.
Blumstein, D. T. & J. C. Daniel, 2005. The loss of anti-predator behaviour following isolation on islands. Proceedings of the Royal Society B-Biological Sciences 272(1573): 1663–1668.
Bray, J. R. & J. T. Curtis, 1957. An ordination of the upland forest communities of southern Wisconsin. Ecological Monographs 27(4): 325–349.
Brechbühl, R., J. Casas & S. Bacher, 2011. Diet choice of a predator in the wild: overabundance of prey and missed opportunities along the prey capture sequence. Ecosphere 2(12):art133.
Brown, J. S., J. W. Laundre & M. Gurung, 1999. The ecology of fear: Optimal foraging, game theory, and trophic interactions. Journal of Mammalogy 80(2): 385–399.
Bruno, J. F., J. J. Stachowicz & M. D. Bertness, 2003. Inclusion of facilitation into ecological theory. Trends in Ecology & Evolution 18(3): 119–125.
Burkholder, D. A., M. R. Heithaus, J. W. Fourqurean, A. Wirsing & L. M. Dill, 2013. Patterns of top-down control in a seagrass ecosystem: could a roving apex predator induce a behaviour-mediated trophic cascade? Journal of Animal Ecology 82(6): 1192–1202.
Burley, L. A., A. T. Moyer & J. W. Petranka, 2006. Density of an intraguild predator mediates feeding group size, intraguild egg predation, and intra- and interspecific competition. Oecologia 148(4): 641–649.
Carter, S. K., G. R. VanBlaricom & B. L. Allen, 2007. Testing the generality of the trophic cascade paradigm for sea otters: a case study with kelp forests in northern Washington, USA. Hydrobiologia 579: 233–249.
Chargulaf, C. A., N. C. Kruck & I. R. Tibbetts, 2011a. Does sympatry affect trophic resource use in congeneric tidepool fishes? A tale of two gobies Favonigobius lentiginosus and Favonigobius exquisitus. Journal of Fish Biology 79(7): 1968–1983.
Chargulaf, C. A., K. A. Townsend & I. R. Tibbetts, 2011b. Community structure of soft sediment pool fishes in Moreton Bay, Australia. Journal of Fish Biology 78(2): 479–494.
Chargulaf, C. A., D. D. Burfeind & I. R. Tibbetts, 2013. A sand goby realizes its niche both at high population densities and in the presence of the half bridled goby. Marine Ecology Progress Series 488: 247–254.
Connell, J. H., 1961. The influence of interspecific competition and other factors on the distribution of the barnacle Chthamalus stellatus. Ecology 42(4): 710–723.
Coull, B. C., J. G. Greenwood, D. R. Fielder & B. A. Coull, 1995. Subtropical Australian juvenile fish eat meiofauna: experiments with winter whiting Sillago maculata and observations on other species. Marine Ecology Progress Series 125: 13–19.
Dukas, R. & A. C. Kamil, 2000. The cost of limited attention in blue jays. Behavioral Ecology 11(5): 502–506.
Farina, S., R. Arthur, J. F. Pages, P. Prado, J. Romero, A. Verges, G. Hyndes, K. L. Heck Jr., S. Glenos & T. Alcoverro, 2014. Differences in predator composition alter the direction of structure-mediated predation risk in macrophyte communities. Oikos 123(11): 1311–1322.
Ferrari, M. C. O., A. Sih & D. P. Chivers, 2009. The paradox of risk allocation: a review and prospectus. Animal Behaviour 78(3): 579–585.
Ferreiro, N., C. Feijoó, A. Giorgi & J. Rosso, 2014. Macroinvertebrates select complex macrophytes independently of their body size and fish predation risk in a Pampean stream. Hydrobiologia 740(1): 191–205.
Fuller, R. A., S. Bearhop, N. B. Metcalfe & T. Piersma, 2013. The effect of group size on vigilance in Ruddy Turnstones arenaria interpres varies with foraging habitat. Ibis 155(2): 246–257.
Giere, O., 2008. Meiobenthology: the microscopic motile fauna of aquatic sediments. Springer Science & Business Media, Berlin.
Gilby, B. L., D. D. Burfeind & I. R. Tibbetts, 2011. Lyngbya majuscula blooms and the diet of small subtropical benthivorous fishes. Marine Biology 158(2): 245–255.
Gilby, B. L., I. R. Tibbetts, A. D. Olds, P. S. Maxwell & T. Stevens, 2016. Seascape context and predators override water quality effects on inshore coral reef fish communities. Coral Reefs 35(3): 979–990.
Hart, S. P. & D. J. Marshall, 2013. Environmental stress, facilitation, competition, and coexistence. Ecology 94(12): 2719–2731.
Hirvonen, H. & E. Ranta, 1996. Prey to predator size ratio influences foraging efficiency of larval Aeshna juncea dragonflies. Oecologia 106(3): 407–415.
Hixon, M. A. & G. P. Jones, 2005. Competition, predation, and density-dependent mortality in demersal marine fishes. Ecology 86(11): 2847–2859.
Hovel, K. A., 2003. Habitat fragmentation in marine landscapes: relative effects of habitat cover and configuration on juvenile crab survival in California and North Carolina seagrass beds. Biological Conservation 110(3): 401–412.
Ingolfsson, A., 2005. Community structure and zonation patterns of rocky shores at high latitudes: an interocean comparison. Journal of Biogeography 32(1): 169–182.
Kaby, U. & J. Lind, 2003. What limits predator detection in blue tits (Parus caeruleus): posture, task or orientation? Behavioral Ecology and Sociobiology 54(6): 534–538.
Kawabata, K., 1991. Ontogenetic changes in copepod behaviours- an ambush cyclopoid predator and a calanoid prey. Journal of Plankton Research 13(1): 27–34.
Knight, T. M., J. M. Chase, C. W. Goss & J. J. Knight, 2004. Effects of interspecific competition, predation, and their interaction on survival and development time of immature Anopheles quadrimaculatus. Journal of Vector Ecology 29(2): 277–284.
Krueck, N. C., C. A. Chargulaf, U. Saint-Paul & I. R. Tibbetts, 2009. Early post-settlement habitat and diet shifts and the nursery function of tidepools during Sillago spp. recruitment in Moreton Bay, Australia. Marine Ecology Progress Series 384: 207–219.
Krueck, N. C., I. R. Tibbetts, R. D. Ward, J. W. Johnson, W. K. W. Loh & J. R. Ovenden, 2013. Multi-gene barcoding to discriminate sibling species within a morphologically difficult fish genus (Sillago). Fisheries Research 143: 39–46.
Mangubhai, S., J. G. Greenwood & I. R. Tibbetts, 1998. Meiofaunal selectivity in the diet of juvenile whiting, (Sillago maculata Quoy and Gaimard), from Moreton Bay. In Tibbetts, I. R., N. J. Hall & W. C. Dennison (eds), Moreton Bay and Catchment. The University of Queensland, Brisbane, Australia, School of Marine Sciences: 473–474.
McHich, R., A. Bergam & N. Raissi, 2005. Effects of density dependent migrations on the dynamics of a predator prey model. Acta Biotheoretica 53(4): 331–340.
R Core Team, 2015. R: A language and environment for statistical computing. In: R Foundation for Statistical Computing, Vienna, Austria. http://www.r-project.org/.
Ripple, W. J. & R. L. Beschta, 2007. Restoring Yellowstone’s aspen with wolves. Biological Conservation 138(3–4): 514–519.
Rushton, S. P., G. W. Barreto, R. M. Cormack, D. W. Macdonald & R. Fuller, 2000. Modelling the effects of mink and habitat fragmentation on the water vole. Journal of Applied Ecology 37(3): 475–490.
Stier, A. C., S. W. Geange & B. M. Bolker, 2013. Predator density and competition modify the benefits of group formation in a shoaling reef fish. Oikos 122(2): 171–178.
White, J. W. & R. R. Warner, 2007. Behavioral and energetic costs of group membership in a coral reef fish. Oecologia 154(2): 423–433.
Worischka, S., C. Hellmann, T. U. Berendonk & C. Winkelmann, 2014. Fish predation can induce mesohabitat-specific differences in food web structures in small stream ecosystems. Aquatic Ecology 48(4): 367–378.
Zaret, T. M. & A. S. Rand, 1971. Competition in tropical stream fishes: support fo the competitive exclusion principle. Ecology 52(2): 336–342.
Acknowledgements
The authors acknowledge the outstanding contribution of the staff at The University of Queensland’s Moreton Bay Research Station in assisting in the set-up and maintenance of the above-described experiments. We thank A. Vazquez, B. Dunlevy, S. Thompson and X. El-Sayed for their assistance in completing experiments. This work was carried out as part of the University of California’s Education Abroad Program (UCEAP) in Australia in the austral winter of 2013. The authors acknowledge the assistance by the study abroad staff at both the University of Queensland and UCEAP.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Luiz Carlos Gomes
Rights and permissions
About this article
Cite this article
Gilby, B.L., Tibbetts, I.R., Van Bourg, J. et al. Predator presence alters prey diet composition but not quantity in tide pool fish interactions. Hydrobiologia 795, 257–265 (2017). https://doi.org/10.1007/s10750-017-3133-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-017-3133-3