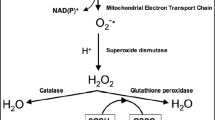
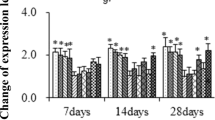
Abstract
Hoplosternum littorale is an Amazon fish that lives in urban areas surrounded by polluted igarapés, where elevated copper concentrations eventually occur. The central goal of this study was to evaluate the associated effects of high temperature and copper contamination on survival time and biochemical responses of the Amazonian fish species H. littorale. We exposed fish to two nominal dissolved copper concentrations (50 and 500 µg l−1) and combined temperatures of 28 and 34°C. Our findings showed that the combination of these variables affects the survival time of this species. The activity of the biotransformation enzymes ethoxyresorufin-O-deethylase and glutathione-S-transferase showed no alterations in fish within all treatments. The increase of reactive oxygen species and the decrease in potential total antioxidant capacity promoted the imbalance in the antioxidant system. An induction in superoxide dismutase activity occurred in fish exposed to copper concentrations of 50 and 500 µg l−1 at both temperatures, suggesting liver impairments. Thus, we suggest that H. littorale is sensitive to copper, and this sensitivity is increased further with exposure to high temperatures, particularly in the survival time and reactive oxygen species formation of this fish species.




Similar content being viewed by others
References
Amado, L. L., M. L. Garcia, P. B. Ramos, R. F. Freitas, B. Zafalon, J. L. R. Ferreira, J. S. Yunes & J. M. Monserrat, 2009. A method to measure total antioxidant capacity against peroxyl radicals in aquatic organisms: application to evaluate microcystins toxicity. Science of the Total Environment 407: 2115–2123.
Bradford, M. M., 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72: 248–254.
Carvalho, C. S. & M. N. Fernandes, 2006. Effect of temperature on copper toxicity and hematological responses in the neotropical fish Prochilodus scrofa at low and high pH. Aquaculture 251: 109–117.
Clarke, F. E., 1950. Determination of chloride in water improved colorimetric and titrimetric methods. Analytical Biochemistry 22: 553–555.
Cusimano, R. F., D. F. Brakke & G. A. Chapman, 1986. Effects of pH on the toxicities of cadmium, copper, and zinc to steelhead trout (Salmo gairdneri). Canadian Journal of Fisheries and Aquatic Sciences 43: 1497–1503.
Daufresne, M., K. Lengfellner & U. Sommer, 2009. Global warming benefits the small in aquatic ecosystems. Proceedings of the National Academy of Sciences 106: 12788–12793.
Duarte, R. M., A. C. L. Menezes, L. S. Rodrigues, V. M. F. Almeida-Val & A. L. Val, 2009. Copper sensitivity of wild ornamental fish of the Amazon. Ecotoxicology and Environmental Safety 72: 693–698.
Erickson, R. J., D. A. Benoit, V. R. Mattson, H. P. N. Jr & E. N. Leonard, 1996. The effects of water chemistry on the toxicity of copper to fathead minnows. Environmental Toxicology and Chemistry 15: 181–193.
Flohé, L. & F. Ötting, 1984. Superoxide dismutase assays. Methods in Enzymology 105: 93–94.
Frenzilli, G., M. Nigro & B. P. Lyons, 2009. The Comet assay for the evaluation of genotoxic impact in aquatic environments. Mutation Research/Reviews in Mutation Research 681: 80–92.
Geracitano, L., J. M. Monserrat & A. Bianchini, 2002. Physiological and antioxidant enzyme responses to acute and chronic exposure of Laeonereis acuta (Polychaeta, Nereididae) to copper. Journal of Experimental Marine Biology and Ecology 277: 145–156.
Gomiero, A. & A. Viarengo, 2014. Effects of elevated temperature on the toxicity of copper and oxytetracycline in the marine model, Euplotes crassus: a climate change perspective. Environmental Pollution 194: 262–271.
Grosell, M., 2012. Copper. In Wood, C. M., A. P. Farrel & C. J. Brauner (eds), Homeostasis and Toxicology of Essential Metals. Academic Press, San Diego: 54–110.
Gustafsson, J. P., 2010. Visual MINTEQ (version 3.0). Department of Land and Water Resources Engineering. The Royal Institute of Technology, Stockholm.
Hoorn, C., F. P. Wesselingh, M. A. Bermudez, A. Mora, I. Sanmartín, A. Sanchez-Meseguer, C. L. Anderson, J. P. Figueiredo, C. Jaramillo, D. Riff, F. R. Negri, H. Hooghiemstra, J. Lundberg, T. Stadler, T. Särkinen & A. Antonelli, 2010. Amazonia through time: Andean uplift, climate change, landscape evolution, and biodiversity. Science 330: 927–931.
Hodson, P. V., P. J. Kloepper-Sams, K. R. Munkittrick, W. L. Lockhart, D. A. Metner, P. L. Luxon, I. R. Smith, M. M. Gagnon, M. Servos & J. F. Payne, 1991. Protocols for measuring mixed function oxygenases of fish livers. Canadian Technical Report of Fisheries and Aquatic Sciences 1829: 33–49.
IPCC, 2013. Fifth Assessment Report on Climate Change 2013: The physical science basis, final draft underlying scientific-technical assessment. Intergovernmental Panel on Climate Change. Working Group 1, Geneva.
Jiang, Z. Y., A. C. S. Woollard & S. Wolf, 1991. Lipid hydroperoxide measurement by oxidation of Fe2+ in the presence of xylenol orange – comparison with the TBA assay and an iodometric method. Lipids 26: 853–856.
Jiang, W. D., Y. Liu, K. Hu, J. Jiang, S. H. Li, L. Feng & X. Q. Zhou, 2014. Copper exposure induces oxidative injury, disturbs the antioxidant system and changes the Nrf2/ARE (CuZnSOD) signaling in the fish brain: protective effects of myo-inositol. Aquatic Toxicology 155: 301–313.
Jomova, K., S. Baros & M. Valko, 2012. Redox active metal-induced oxidative stress in biological systems. Transition Metal Chemistry 37: 127–134.
Jones, D. P., 2006. Redefining oxidative stress. Antioxidants & Redox Signaling 8: 1865–1879.
Keen, J. H., W. H. Habig & W. B. Jakoby, 1976. Mechanism for the several activities of the glutathione-S-transferase. Journal of Biological Chemistry 251: 6183–6188.
Kehrer, J. P., 2000. The Haber–Weiss reaction and mechanisms of toxicity. Toxicology 149: 43–50.
Lee, S., K. Ji & K. Choi, 2014. Effects of water temperature on perchlorate toxicity to the thyroid and reproductive system of Oryzias latipes. Ecotoxicology and Environmental Safety 108: 311–317.
Lushchak, V. I., 2011. Environmentally induced oxidative stress in aquatic animals. Aquatic Toxicology 101: 13–30.
Lushchak, V. I. & T. V. Bagnyukova, 2006. Temperature increase results in oxidative stress in goldfish tissues. 1. Indices of oxidative stress. Comparative Biochemistry and Physiology, Part C 143: 30–35.
Matsuo, A. Y. O., R. C. Playle, A. L. Val & C. M. Wood, 2004. Physiological action of dissolved organic matter in rainbow trout in the presence and absence of copper: sodium uptake kinetics and unidirectional flux rates in hard and softwater. Aquatic Toxicology 70: 63–81.
Matsuo, A. Y. O., C. M. Wood & A. L. Val, 2005. Effects of copper and cadmium on ion transport and gill metal binding in the Amazonian teleost tambaqui (Colossoma macropomum) in extremely soft water. Aquatic Toxicology 74: 351–364.
Monteiro, S. M., N. Santos, M. Calejo, A. Fontainhas-Fernandes & M. Sousa, 2009. Copper toxicity in gills of the teleost fish, Oreochromis niloticus: effects in apoptosis induction and cell proliferation. Aquatic Toxicology 94: 219–228.
Mora, C. & M. F. Moya, 2006. Effect of the rate of temperature increase of the dynamic method on the heat tolerance of fishes. Journal of Thermal Biology 31: 337–341.
Noyes, P. D., M. K. McElwee, H. D. Miller, B. W. Clark, L. A. Van Tiem, K. C. Walcott, K. N. Erwin & E. D. Levin, 2009. The toxicology of climate change: environmental contaminants in a warming world. Environment International 35: 971–986.
Osman, A. G. M., A. M. Abd El Reheem, K. Y. AbuelFadl & A. G. GadEl-Rab, 2010. Enzymatic and histopathologic biomarkers as indicators of aquatic pollution in fishes. Natural Science 2: 1302–1311.
Playle, R. C., D. G. Dixon & K. Burnissn, 1993. Copper and cadmium binding to fish gills: estimates of metal-gill stability constants and modelling of metal accumulation. Canadian Journal of Fisheries and Aquatic Sciences 50: 2667–2677.
Pottinger, T. G., T. R. Carrick & W. E. Yeomans, 2002. The three-spined stickleback as an environmental sentinel: effects of stressors on whole-body physiological indices. Journal Fish Biology 61: 207–229.
Richards, J. G., B. K. Burnison & R. C. Playle, 1999. Natural and commercial dissolved organic matter protects against the physiological effects of a combined cadmium and copper exposure on rainbow trout (Oncorhynchus mykiss). Canadian Journal of Fisheries and Aquatic Sciences 56: 407–418.
Rose, W. L., R. M. Nisbet, P. G. Green, S. Norris, T. Fan, E. H. Smith, G. N. Cherr & S. L. Anderson, 2006. Using an integrated approach to link biomarker responses and physiological stress to growth impairment of cadmium-exposed larval topsmelt. Aquatic Toxicology 80: 298–308.
Sanchez, W., O. Palluel, L. Meunier, M. Coquery, J. M. Porcher & S. Ait-Aıssa, 2005. Copper-induced oxidative stress in three-spined stickleback: relationship with hepatic metal levels. Environmental Toxicology and Pharmacology 19: 177–183.
Schiedek, D., B. Sundelin, J. W. Readman & R. W. Macdonald, 2007. Interactions between climate change and contaminants. Marine Pollution Bulletin 54: 1845–1856.
Sokolova, I. M. & G. Lanning, 2008. Interactive effects of metal pollution and temperature on metabolism in aquatic ectotherms: implications of global climate change. Climate Research 37: 181–201.
Straile, D., D. M. Livingstone, G. A. Weyhenmeyer & D. G. George, 2013. The response of freshwater ecosystems to climate variability associated with the North Atlantic Oscillation. In: The North Atlantic Oscillation: Climatic Significance and Environmental Impact, pp. 263–279.
Stohs, S. J. & D. Bagchi, 1995. Oxidative mechanisms in the toxicity of metal ions. Free Radical Biology and Medicine 18: 321–336.
Valko, M. M. H. C. M., H. Morris & M. T. D. Cronin, 2005. Metals, toxicity and oxidative stress. Current Medicinal Chemistry 12: 1161–1208.
Van der Oost, R., J. Beyer & N. P. E. Vermeulen, 2003. Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environmental Toxicology and Pharmacology 13: 57–149.
Vieira, L. R., C. Gravato, A. M. V. M. Soares, F. Morgado & L. Guilhermino, 2009. Acute effects of copper and mercury on the estuarine fish Pomatoschistus microps: linking biomarkers to behavior. Chemosphere 76: 1416–1427.
Wang, T., X. Long, Y. Cheng, Z. Liu & S. Yan, 2014. The potential toxicity of copper nanoparticles and copper sulphate on juvenile Epinephelus coioides. Aquatic Toxicology 152: 96–104.
Welsh, P. G., J. F. Skidmore, D. J. Spry, D. G. Dixon, P. V. Hodson, N. J. Hutchinson & B. E. Hickie, 1993. Effect of pH and dissolved organic carbon on the toxicity of copper to larval fathead minnow (Pimephales promelas) in natural lake waters of low alkalinity. Canadian Journal of Fisheries and Aquatic Sciences 50: 1356–1362.
Wood, C. M., H. A. Al-Reasi & D. S. Smith, 2011. The two faces of DOC. Aquatic Toxicology 105S: 3–8.
Worms, I., D. F. Simon, C. S. Hassler & K. J. Wilkinson, 2006. Bioavailability of trace metals to aquatic microorganisms: importance of chemical, biological and physical processes on biouptake. Biochimie 88: 1721–1731.
Yu-Ting Lim, M., A. M. Zimmer & C. M. Wood, 2015. Acute exposure to waterborne copper inhibits both the excretion and uptake of ammonia in freshwater rainbow trout (Oncorhynchus mykiss). Comparative Biochemistry Physiology Part C 168: 48–54.
Zar, J. K., 1984. Biostatistical Analysis. Prentice-Hall, New Jersey.
Acknowledgments
We acknowledge the joint grant from the Brazilian National Research Council (CNPq) and Amazonas State Research Foundation (FAPEAM) to ADAPTA Project (Environmental Adaptations of Aquatic Organisms) that supported this work. We thank Maria de Nazaré Paula-Silva for all support during the ADAPTA expedition.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: Adalberto L. Val, Gudrun De Boeck & Sidinei M. Thomaz / Adaptation of Aquatic Biota of the Amazon
Rights and permissions
About this article
Cite this article
Braz-Mota, S., Fé, L.M.L., Delunardo, F.A.C. et al. Exposure to waterborne copper and high temperature induces the formation of reactive oxygen species and causes mortality in the Amazonian fish Hoplosternum littorale . Hydrobiologia 789, 157–166 (2017). https://doi.org/10.1007/s10750-016-2847-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-016-2847-y