Abstract
Stoichiometric theory predicts that organisms should experience dietary imbalances not only when nutrients (e.g., phosphorus, P) are limiting relative to carbon (C), but also when nutrients are in excess (i.e., well above somatic demand). Nevertheless, few experiments have elucidated the response of consumers in such low C:P conditions. We assessed the growth, tissue stoichiometry, and nutrient excretion of the invasive primary consumer, zebra mussel (ZM), Dreissena polymorpha, under three dietary C:P conditions (C:P = 20, 45, 380) in the laboratory. The two low C:P conditions represent increasingly common eutrophic systems, while the high C:P treatment is representative of oligotrophic systems. Growth rates and condition were lower when ZMs were fed a low C:P (20 and 45) diet, compared to the C:P = 380 treatment, wherein ZMs grew rapidly and exhibited lower somatic C:P. Furthermore, ZMs in the C:P = 20 and C:P = 45 treatments excreted more ammonia indicative of protein catabolism. These results clearly show that hypereutrophic conditions invoke significant shifts in physiology, growth, and condition of ZMs. Together, these results are consistent with stoichiometric theory that predicts costs associated with the intake of excess dietary P.
Similar content being viewed by others
Introduction
Elemental imbalances occur when consumers obtain food items that contain essential elements in proportions that are different from the proportions required for growth, reproduction, and maintenance (Sterner & Elser, 2002). Such stoichiometric imbalances between consumers and their food resources are common in most ecosystems, and influence the performance and distribution of species (Sterner & Elser, 2002). Imbalances are particularly strong at the base of foodwebs where the stoichiometry of primary producers is often different from primary consumers (Elser et al., 2000), leading to stoichiometric constraints on consumer growth and performance, despite constant food (energy) supply. Primary consumers grow optimally when the elemental composition of the food resource is balanced with respect to the consumer’s dietary requirements (Sterner & Hessen, 1994). In many ecosystems, carbon is often in surplus while nutrients are limiting (Hessen & Anderson, 2008). Thus, foods with high carbon (C, energy) content and low nutrient content are regarded as low quality, while higher concentrations of mineral nutrients indicate high food quality.
Organismal performance along most nutritional axes follows a unimodal relationship (Fig. 1; Karasov & Martinez del Rio, 2007). Thus, low food quality can also be associated with food resources that have an excess of nutrients. Anthropogenic activities have led to a several-fold increase in bioavailable phosphorus (P) in the last century (Smil, 2000), and have made information on the consequences of excess dietary P in organisms relevant even in natural systems (Fig. 1; Sterner et al., 2008). Anthropogenic sources of nutrients have resulted in the eutrophication of hundreds of lakes in the last fifty years (Schindler, 2006). Clearly, studies examining the effects of excess nutrients on the growth and condition of predominant consumers in ecosystems are needed (Smith & Schindler, 2009).
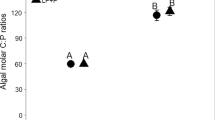
Histogram of freshwater lakes and ponds worldwide and seston C:P ratios from Sterner et al. (2008). The dashed vertical line represents seston C:P ratio from Sooner Lake, Oklahoma, where zebra mussels used in this study were collected. Solid vertical line represents the estimated zebra mussel C:P threshold elemental ratio based on Frost et al. (2006). The dashed curve represents an approximation of the growth response predicted by stoichiometric theory when zebra mussels are fed seston with varying C:P
The invasive zebra mussel (ZM) (Dreissena polymorpha), is a dominant consumer that has altered the structure and function of aquatic communities in North America since their invasion in the late 1980s (Strayer, 2009). While its ecological impacts are well known, little is known about the physiology of ZMs that underlie such striking ecological consequences (Liess & Hillebrand, 2005; Naddafi et al., 2009). Recent studies indicate that ZMs alter their tissue stoichiometry in response to varying degrees of nutrient limitation (Liess & Hillebrand, 2005; Naddafi et al., 2008, 2009). Theory predicts that organisms capable of altering somatic stoichiometry in response to environmental supply should have a competitive advantage over those that are incapable of integrating environmental variation into somatic composition (Hood & Sterner, 2010), and increase the likelihood of invasions (Gonzalez et al., 2010).
To our knowledge, nothing is known about the performance of ZMs in eutrophic to hyper-eutrophic conditions where seston C:P is considerably lower than those observed in oligo/mesotrophic lakes. Here, we examined how phosphorus-rich algae affected ZM growth, tissue stoichiometry, and nutrient excretion. Specifically, we tested the effects of low seston C:P typical of hyper-eutrophic systems provided at two quantities (above and below incipient limiting concentration), on ZM performance. Two of the three C:P treatments selected in this study fall within the lower fifteen percent of seston C:P (C:P < 100) found in freshwater lakes and ponds worldwide, while the third treatment is similar to ratios in the upper ten percent (Fig. 1; C:P >380; Sterner et al., 2008). We predicted that ZM growth will be lower when feeding on algae with very low C:P; and as a result, based on the growth rate hypothesis (Elser et al., 1996) somatic C:P should be higher. Moreover, we quantified the excretion of P as soluble reactive phosphorus, and nitrogen as ammonia by ZMs to better understand variation in P use, and overall metabolic status of experimental animals. Based on stoichiometric theory (Sterner & Elser, 2002) we predicted that P excretion will be higher in low C:P conditions. We also predicted that ammonia excretion will be higher because there is evidence that consumers may become energy starved (i.e., experience net catabolism) when feeding on resources with very low C:P ratios (Boersma & Elser, 2006).
Methods
Generation of algae with contrasting C:P stoichiometry
Scenedesmus obliquus was cultured in semi-continuous three-liter chemostats on a standardized medium (Kilham et al., 1998) differing in P content (1.55 × 103, 4.355 × 106, and 4.355 × 107 μg P l−1) under a 16:8 (light:dark) cycle. S. obliquus was allowed to grow until the chemostats reached stable state. Harvested algae were stored at 4°C until enough algae was accumulated for the experiment (approximately 1 week). All algae of a given treatment were combined into one large container and thoroughly homogenized. Ten aliquots were sub-sampled for estimation of C and P. Algae were filtered onto acid washed and combusted (550°C) Whatman GF/F filters for estimation of C using an elemental analyzer (Elementar Americas, Inc., Mt. Laurel, New Jersey), while P was estimated using a modified sulfuric acid digestion method (APHA, 1995) and analyzed using a Genesys 20 spectrophotometer, (Thermo Electron Corporation, Madison, Wisconsin).
Experimental set-up
Due to logistical constraints, this experiment was conducted over two summers (2010 and 2011). In the 2010 trial, ZMs were exposed for 30 days to two food qualities (C:P = 20 and C:P = 45), representative of hypereutrophic conditions, and one of two levels of food quantity (1 mg C l−1 and 4 mg C l−1 day−1, above and below incipient level, respectively; Walz, 1978). In 2011, mussels were exposed to C:P = 380 diet at either 1 mg or 4 mg C l−1 day−1 representative of meso-/oligotrophic conditions.
Zebra mussel druses (approximately 300 individuals in total) of similar size (7–9 mm) were collected from Sooner Reservoir, Oklahoma (36°24′30″N, 97°01′17″W). The seston C:P stoichiometry of Sooner Reservoir at the time of ZM collection was 46.2 ± 4.33 (N = 5; mean ± 1 SD). Mussel druses were collected and placed in an insulated container filled with lake water and transported back to the laboratory. Individual mussels located on the outer edges of the druses were separated by cutting the byssal threads with a scalpel and acclimated to laboratory conditions (23°C ± 1) in aerated containers for 2 days.
Zebra mussel threshold elemental ratio (TER) was estimated to be ~260 based on Frost et al. (2006). This model assumes strict homeostasis, while ZMs are known to be nonhomeostatic (Naddafi et al., 2009). While additional parameters are needed for a precise estimate of ZM C:P TER, we exposed ZMs to dietary conditions well below estimated TER (i.e., C:P = 20 and C:P = 45), and considerably above estimated TER (i.e., C:P = 380). As such, our design is useful to test whether excess P in food resources affect ZM growth and performance.
Trials were conducted in 1-L microcosms filled with 800 mL of de-chlorinated tap water under a 16:8 light:dark cycle and held at 23°C ± 1 for 30 days. Food (carbon) concentration of Scenedesmus algae was determined spectrophotometrically following equations generated from several years of direct absorbance (at 660 and 750 nm) and %C measurements (Jeyasingh PD, unpublished data). Each dietary treatment (i.e., two levels of food quantity, and three levels of food C:P) was replicated 15 times. Complete water changes, using de-chlorinated tap water were performed every other day.
Growth and tissue stoichiometry
Dry mass and shell length were measured to the nearest 0.001 mg using a Mettler-Toledo ultra-microbalance (Mettler-Toledo XP2U, Columbus, OH), and 0.01 mm using electronic digital calipers (Fisher Scientific, Pittsburgh, Pennsylvania), respectively. Pre- and post-experimental shell length measurements were used to calculate ZM growth over the duration of the experiment. After measurement of shell length, soft tissue was removed using a scalpel. Soft tissue and shells were dried at 60°C for 48 h. Percent C of soft tissue was determined using an elemental analyzer, and % P was analyzed using a modified sulfuric acid digestion method (APHA, 1995). Tissue from the same individual was used for estimation of molar C and P. A subsample of 5 mussels from each sampling date was used for determination of initial tissue condition index, dry tissue mass, shell mass, and tissue stoichiometry.
Nutrient excretion
After experimental exposure to various dietary treatments, the individual ZM was removed from the microcosm and placed in 200 mL of fresh de-chlorinated tap water for 3 h for measurement of nutrient excretion rates. After 3 h, ZMs were removed for estimation of tissue stoichiometry (see above), and the water was immediately analyzed for ammonia (USEPA Method 350.1; Fisher Scientific Accumet excel XL25 Dual Channel pH/Ion Meter, Pittsburgh, PA). Soluble reactive phosphorus was estimated spectrophotometrically (APHA, 1995). Five containers without ZMs were used as controls. Mean ammonia and soluble reactive phosphorus levels in these containers were measured and subtracted from the values obtained after ZM incubation in experimental containers to quantify ZM excretion.
Statistics
Since the experiment included two discrete trials (2010 and 2011), the data should be analyzed using a three-way ANOVA with year, food quantity, and food quality as fixed factors. However, we did not perform all three food quality treatments in both years, precluding the isolation of year effects from food quality effects. Thus, we first used a one-way ANOVA to test for year specific variation in the initial tissue stoichiometry, tissue condition index, and shell length of the ZMs that were used in the two trials. Since the initial conditions of ZMs did not differ (see Results below), we used two-way ANOVAs to determine whether food quality and food quantity treatments affected ZM growth, tissue stoichiometry, and nutrient excretion for each of the C:P treatments (Sigma Stat 3.5; Systat Software, Inc., Richmond, California, USA). Data (C, N, and P values) were log10 transformed to meet the assumptions of normality. Tukey’s (HSD) post hoc comparisons (P < 0.05) were used when ANOVAs were significant.
Results
Experimental manipulation of P supply in chemostats resulted in algae differing significantly in C:P stoichiometry (F2,9 = 557.186, P < 0.001). Initial ZM tissue stoichiometry (N = 5 for each trial; C:P, F1,9 = 2.456, P = 0.156; Table 1), tissue condition index (F1,9 = 3.750, P = 0.095), and ZM length (F1,9 = 3.34, P = 0.647) did not vary significantly between trials conducted in 2010 and 2011.
Food quantity effects
At the end of trials, food quantity had no effect on ZM growth, tissue stoichiometry, and nutrient excretion in both trials (P > 0.05 for all comparisons). This result indicates that the published incipient limiting concentration of 1 mg C l−1 day−1 (Walz, 1978) is above actual incipient limiting concentration for ZMs from Sooner Lake. Therefore, we focus only on the results pertaining to food quality at high food quantity (4 mg C l−1 day−1) representative of hypereutrophic lakes (Wetzel, 2001). Percent nutrients (C, N, and P) and molar ratios (C:P) for ZMs from each treatment are presented in Table 1.
Food quality effects on tissue stoichiometry, growth rate, and condition index
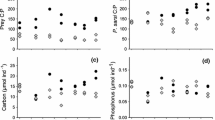
Food quality significantly affected tissue C:P (F2,77 = 21.787, P < 0.001), C:N (F2,77 = 18.772, P < 0.001), and N:P (F2, 77 = 30.310, P < 0.001). Specifically, tissue C:P was significantly lower in the C:P = 380 treatment than the C:P = 20 and C:P = 45 treatments (Fig. 2). Zebra mussel shell length varied significantly with food quality (F2,77 = 40.997, P < 0.001). Shell growth (mean ± 1 SD) rate was 0.001 ± 0.002 mm day−1 in the C:P = 20 treatment, 0.005 ± 0.003 mm day−1 in the C:P = 45 treatment, while ZMs under C:P = 380 grew at a rate of 0.0169 ± 0.003 mm day−1 (Fig. 3A). Post-hoc tests revealed that the two low treatments (C:P = 20 and 45) collectively differed from the C:P = 380 treatment. Food quality also had a significant effect on dry tissue mass (F2,77 = 65.582, P < 0.001). Zebra mussels in the C:P = 380 treatments increased their dry tissue mass values while the mussels in the C:P = 20 or C:P = 45 treatments had decreased dry tissue mass values relative to the initial dry tissue mass (Fig. 3B).
Food quality effects on SRP and NH3 excretion
Soluble reactive phosphorus excretions were significantly different between mussels grown in the three food quality treatments (F2,77 = 50.942, P < 0.001). Clearly, excretions were higher in the C:P = 20 treatments than they were in the C:P = 45 and C:P = 380, and the lowest under C:P = 380 treatments (Fig. 4A). Ammonia excretion rates were also significantly affected by the three food quality treatments (F2,77 = 65.515, P < 0.001), with mussels grown in the C:P = 20 treatments excreting more than those grown in the C:P = 45 and C:P = 380 treatments, while ammonia excretion was the lowest under C:P = 380 (Fig. 4B).
Discussion
We found strong negative effects of excessive dietary P (i.e., low C:P diet) on ZM growth. Specifically, ZMs grown in the C:P = 20 and C:P = 45 treatments grew slower than ZMs in the C:P = 380 treatment. Moreover, ZMs in low C:P conditions excreted more P and ammonia than ZMs in the C:P = 380 treatment. Note that although we were unable to compare the effects of food quality on response variables between the trials conducted in 2010 and 2011 in a single model, initial ZM parameters (i.e., growth, stoichiometry, condition) did not differ between years. Moreover, food quantity effects on all measured parameters did not vary between years. The striking differences in growth responses to dietary stoichiometry (Fig. 3) indicate that excessive dietary P constrains ZM production, and affects key ecological functions such as nutrient excretion (Fig. 4).
Boersma & Elser (2006) reviewed a handful of studies that examined the effects of food with high P content on consumer growth and found significant negative effects, indicating costs associated with excess dietary P intake. Both behavioral and physiological mechanisms have been invoked to explain reduced growth in response to excess P (Elser et al., 2005). Plath & Boersma (2001) found that Daphnia decreased feeding when presented food with excessive P. Such behavioral responses can invoke growth and condition penalties, because individuals starve themselves for energy after P requirements are met despite the presence of abundant food.
ZMs may also exhibit such reductions in feeding rates, but lack of data on food consumption rates precludes us from ascertaining this. On the other hand, ZMs may exhibit compensatory feeding (i.e., elevated feeding) in response to stoichiometric imbalances, resulting in food quantity limitation and potentially obscuring our food quality (i.e., C:P) effects. However, we purposefully supplied food at much higher quantities (i.e., 4 mg C l−1) than the reported incipient limiting food level for ZMs (2 mg C l−1; Walz, 1978). Moreover, our results indicate that limiting food concentrations are lower than 2 mg C l−1. Thus, it is highly unlikely that ZMs in controlled experimental conditions (i.e., density) become energy limited at such high food supply conditions.
Physiological responses to excessive dietary P include decreased assimilation efficiency, inhibitory interactions with the transport of micronutrients, and alteration of several metabolic substrates (Silber et al., 2002). For example, excess dietary P will likely alter P concentration gradients across the membrane of gut epithelial cells and thus could disrupt symporter (e.g., Na-dependent P transporters) functions (Karasov & Martinez del Rio, 2007). Restoring Na balance requires energy and could be a major cost associated with excess P. While further work is needed to illuminate the physiological mechanisms underlying striking shifts in growth, condition, and nutrient excretion, these observations clearly indicate that food with high P concentrations constrain ZM performance.
Data show that ZMs tissue C:P significantly decreased in all treatments relative to initial conditions (Table 1). Nevertheless, we found that ZMs in the C:P = 380 treatment have lower C:P indicative of rapid growth (Elser et al., 1996) compared to C:P = 20 and C:P = 45 treatments. Similar to studies in oligo- and meso-trophic (i.e., high seston C:P ratios) conditions (Liess & Hillebrand, 2005; Naddafi et al., 2009), our data indicate that under stoichiometric imbalances driven by excess nutrients (i.e., very low seston C:P ratios) ZMs are not homeostatic in their tissue stoichiometry. However, contrary to observations from oligo-/mesotrophic conditions (Naddafi et al., 2009), such low dietary C:P does invoke stoichiometric constraints, because ZMs in the C:P = 20 and C:P = 45 treatments did not grow (C:P = 45) or loose mass (C:P = 20). Specifically, Naddafi et al. (2009) found that stoichiometric constraints did not limit the invasion success and range expansion of ZMs in two Swedish lakes that had an average seston C:P = 160, our observations in dietary conditions well below the threshold elemental ratio of ~260 (Frost et al., 2006) indicate that ZMs may be susceptible to stoichiometric imbalances when P is in excess.
Shifts in growth and condition were reflected in ZM somatic stoichiometry, and nutrient excretion. We found that ZMs in the C:P = 380 treatment had on average 1.63% P in their tissues while ZMs from the C:P = 20 and C:P = 45 treatments had 0.46% and 0.58%, respectively. Moreover, P excretion was higher in the C:P = 20 and C:P = 45 treatments compared to the C:P = 380 treatment. Stanczykowska & Lewandowski (1993) report that during periods that are favorable for growth, ZMs can accumulate considerable amounts of P in their tissue. It is likely that P accumulated in ZMs is excreted when ZMs undergo net catabolism under unfavorable conditions such as very high dietary P. Indeed, James (2001) found that tissue emaciation during periods of energetic stress such as declines in food quality and quantity result in the release of P by ZMs back into the water column. Nutrient recycling by ZMs has been evaluated at multiple levels with mixed results depending on algal community structure and difference between natural systems and reservoirs (Higgins & Vander Zanden, 2010; Kirsch & Dzialowski, 2012). Our results suggest that the effects may be context dependent depending on initial nutrient concentrations in invaded systems. Additionally, Fig. 4 indicates that ZMs perform such catabolic processes in the C:P = 20 and C:P = 45 treatments with higher excretion rates of ammonia, an indicator of net catabolism (Wilmer, 2005), compared to C:P = 380. Together, these results are strongly indicative of costs associated with the intake of excess P in ZMs.
Our data clearly indicate that excessive dietary P impacts ZM growth, tissue stoichiometry, and nutrient excretion. As pointed out by Naddafi et al. (2009), ZMs have low P requirements relative to native taxa, and yet sustain high growth rates, indicating high P use efficiency. While low P requirements may aid in mitigating stoichiometric imbalances when nutrient are limiting, they may make ZMs more vulnerable to excess dietary P, a tradeoff found in a family of P-efficient plants (Hawkins et al., 2008). Indeed, studies have shown that ZM density and biomass are negatively correlated with phosphorus loading (e.g., Naddafi et al., 2011). While most studies in ecological stoichiometry have focused on conditions where nutrients are limiting (Boersma & Elser, 2006), understanding the responses of organisms in conditions where nutrients are in excess is becoming increasingly important as cultural eutrophication proceeds at a rapid pace. Such information will be useful to better understand altered ecological functions (e.g., nutrient recycling) of ZMs, and their invasion potential into contrasting ecosystems such as oligotrophic glacial lakes and hypereutrophic reservoirs.
References
APHA, 1995. Standard Methods for the Examinations of Water and Wastewater, 19th ed. American Public Health Association, Washington, DC.
Boersma, M. & J. J. Elser, 2006. Too much of a good thing: on stoichiometrically balanced diets and maximal growth. Ecology 87(5): 1325–1330.
Elser, J. J., D. R. Dobberfuhl, N. A. MacKay & J. H. Schampel, 1996. Organism size, life history, and N:P stoichiometry. Bioscience 46(9): 674–684.
Elser, J. J., W. F. Fagan, R. F. Denno, D. R. Dobberfuhl, A. Folarin, A. Huberty, S. Interlandi, S. S. Kilham, E. McCauley, K. L. Schulz, E. H. Siemann & R. W. Sterner, 2000. Nutritional constraints in terrestrial and freshwater food webs. Nature 408(6812): 578–580.
Elser, J. J., J. H. Schampel, M. Kyle, J. Watts, E. W. Carson, T. E. Dowling, C. Tang & P. D. Roopnarine, 2005. Response of grazing snails to phosphorus enrichment of modern stromatolitic microbial communities. Freshwater Biology 50(11): 1826–1835.
Frost, P. C., J. P. Benstead, W. F. Cross, H. Hillebrand, J. H. Larson, M. A. Xenopoulos & T. Yoshida, 2006. Threshold elemental ratios of carbon and phosphorus in aquatic consumers. Ecology Letters 9(7): 774–779.
Gonzalez, A. L., J. S. Kominoski, M. Danger, S. Ishida, N. Iwai & A. Rubach, 2010. Can ecological stoichiometry help explain patterns of biological invasions? (119, 779–790, 2010). Oikos 119(6):1056–1056.
Hawkins, H. J., H. Hettasch, J. Mesjasz-Przybylowicz, W. Przybylowicz & M. D. Cramer, 2008. Phosphorus toxicity in the Proteaceae: a problem in post-agricultural lands. Scientia Horticulturae 117(4): 357–365.
Hessen, D. O. & T. R. Anderson, 2008. Excess carbon in aquatic organisms and ecosystems: physiological, ecological, and evolutionary implications. Limnology and Oceanography 53(4): 1685–1696.
Higgins, S. N. & M. J. Vander Zanden, 2010. What a difference a species makes: a meta-analysis of dreissenid mussel impacts on freshwater ecosystems. Ecological Monographs 80(2): 179–196.
Hood, J. M. & R. W. Sterner, 2010. Diet mixing: do animals integrate growth or resources across temporal heterogeneity? American Naturalist 176(5): 651–663.
James, W. F., 2001. Phosphorus recycling by zebra mussels in relation to density and food resource availability. Hydrobiologia 455: 55–60.
Karasov, W. H. & C. Martinez del Rio, 2007. Physiological ecology: how animals process energy, nutrients, and toxins. Princeton University Press, Princeton.
Kilham, S. S., D. A. Kreeger, S. G. Lynn, C. E. Goulden & L. Herrera, 1998. COMBO: a defined freshwater culture medium for algae and zooplankton. Hydrobiologia 377: 147–159.
Kirsch, K. M. & A. R. Dzialowski, 2012. Effects of invasive zebra mussels on phytoplankton, turbidity, and dissolved nutrients in reservoirs. Hydrobiologia 686(1): 169–179.
Liess, A. & H. Hillebrand, 2005. Stoichiometric variation in C:N, C:P, and N:P ratios of littoral benthic invertebrates. Journal of the North American Benthological Society 24(2): 256–269.
Naddafi, R., K. Pettersson & P. Eklov, 2008. Effects of the zebra mussel, an exotic freshwater species, on seston stoichiometry. Limnology and Oceanography 53(5): 1973–1987.
Naddafi, R., P. Eklov & K. Pettersson, 2009. Stoichiometric constraints do not limit successful invaders: zebra mussels in Swedish lakes. Plos One 4(4). doi:10.1371/journal.pone.0005345.
Naddafi, R., T. Blenckner, P. Eklov & K. Pettersson, 2011. Physical and chemical properties determine zebra mussel invasion success in lakes. Hydrobiologia 669(1): 227–236.
Plath, K. & M. Boersma, 2001. Mineral limitation of zooplankton: stoichiometric constraints and optimal foraging. Ecology 82(5): 1260–1269.
Schindler, D. W., 2006. Recent advances in the understanding and management of eutrophication. Limnology and Oceanography 51(1): 356–363.
Silber, A., J. Ben-Jaacov, A. Ackerman, A. Bar-Tal, I. Levkovitch, T. Matsevitz-Yosef, D. Swartzberg, J. Riov & D. Granot, 2002. Interrelationship between phosphorus toxicity and sugar metabolism in Verticordia plumosa. Plant Soil 245(2): 249–260.
Smil, V., 2000. Phosphorus in the environment: natural flows and human interferences. Annual Review of Energy and the Environment 25: 53–88.
Smith, V. H. & D. W. Schindler, 2009. Eutrophication science: where do we go from here? Trends in Ecology & Evolution 24(4): 201–207.
Stanczykowska, A. & K. Lewandowski, 1993. Effect of filtering activity of Dreissena-polymorpha (Pall) on the nutrient budget of the littoral of Lake Mikolajskie. Hydrobiologia 251(1–3): 73–79.
Sterner, R. W. & J. J. Elser, 2002. Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton University Press, Princeton.
Sterner, R. W. & D. O. Hessen, 1994. Algal nutrient limitation and the nutrition of aquatic herbivores. Annual Review of Ecology and Systematics 25: 1–29.
Sterner, R. W., T. Andersen, J. J. Elser, D. O. Hessen, J. M. Hood, E. McCauley & J. Urabe, 2008. Scale-dependent carbon:nitrogen:phosphorus seston stoichiometry in marine and freshwaters. Limnology and Oceanography 53(3): 1169–1180.
Strayer, D. L., 2009. Twenty years of zebra mussels: lessons from the mollusk that made headlines. Frontiers in Ecology and the Environment 7(3): 135–141.
Walz, N., 1978. The energy balance of the freshwater mussel Dreissena polymorpha Pallas in laboratory experiments and in Lake Constance. I: pattern of activity, feeding and assimilation efficiency. Archiv in Hydrobiologia Supplement Band 55: 83–105.
Wetzel, R. G., 2001. Limnology: lake and river ecosystems. Academic Press, San Diego, CA.
Wilmer, P., 2005. Environmental physiology of animals, 2nd ed. Blackwell Publishing, Malden, MA.
Acknowledgments
J.R. Bidwell and J.B. Belden are acknowledged for their insight and constructive criticism on a previous version. We thank P.C Frost for help in determining ZM TER values and three reviewers that improved this manuscript. This project was funded in part by Waters Grant-in-Aid of Research Award to RLM, and NSF grant # 0924401 to PDJ.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: John Havel
Rights and permissions
About this article
Cite this article
Morehouse, R.L., Dzialowski, A.R. & Jeyasingh, P.D. Impacts of excessive dietary phosphorus on zebra mussels. Hydrobiologia 707, 73–80 (2013). https://doi.org/10.1007/s10750-012-1407-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-012-1407-3