Abstract
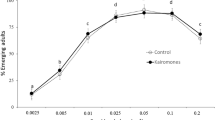
Cyclopoid copepods are important predators in many aquatic ecosystems and have been used as biological agents in successful programs to control mosquito larvae. However, the impacts of this predation on adult mosquito populations are still poorly understood. The present study compared the sex ratios and body sizes (measured as wing length) of Aedes albopictus mosquitoes emerging from recipients containing the copepod predator Mesocyclops ogunnus with control situations without this predator. We found that copepod predation significantly biased mosquito sex ratios toward females, and that both the males and females emerging from copepod-containing recipients were significantly larger than control insects. The ecological and epidemiological consequences of the changes induced by copepod predation on mosquito populations are discussed.



Similar content being viewed by others
References
Acharya, L., 1995. Sex-biased predation on moths by insectivorous bats. Animal Behaviour 49(6): 1461–1468.
Alto, B., M. Griswold & L. Lounibos, 2005. Habitat complexity and sex-dependent predation of mosquito larvae in containers. Oecologia 146(2): 300–310.
Alto, B. W., M. H. Reiskind & L. P. Lounibos, 2008. Size alters susceptibility of vectors to dengue virus infection and dissemination. The American Journal of Tropical Medicine and Hygiene 79(5): 688–695.
Armbruster, P. & R. A. Hutchinson, 2002. Pupal mass and wing length as indicators of fecundity in Aedes albopictus and Aedes geniculatus (Diptera: Culicidae). Journal of Medical Entomology 39(4): 699–704.
Bedhomme, S., P. Agnew, C. Sidobre & Y. Michalakis, 2003. Sex-specific reaction norms to intraspecific larval competition in the mosquito Aedes aegypti. Journal of Evolutionary Biology 16(4): 721–730.
Beserra, E. B., C. R. M. Fernandes & P. S. Ribeiro, 2009. Relação entre densidade larval e ciclo de vida, tamanho e fecundidade de Aedes (Stegomyia) aegypti (L.) (Diptera: Culicidae) em laboratório. Neotropical Entomology 38: 847–852.
Chaves, L. F., C. L. Keogh, A. M. Nguyen, G. M. Decker, G. M. Vazquez-Prokopec & U. D. Kitron, 2011. Combined sewage overflow accelerates immature development and increases body size in the urban mosquito Culex quinquefasciatus. Journal of Applied Entomology 135(8): 611–620.
Devicari, M., A. R. Lopes & L. Suesdek, 2011. Dimorfismo sexual alar em Aedes scapularis (Diptera: Culicidae). Biota Neotropica 11: 165–169.
Forattini, O. P., G. R. A. M. Marques, I. Kakitani, M. de Brito & M. A. M. Sallum, 1998. Significado epidemiológico dos criadouros de Aedes albopictus em bromélias. Revista de Saúde Pública 32: 186–188.
Gama, R. A., K. C. Alves, R. F. Martins, Á. E. Eiras & M. C. Resende, 2005. Efeito da densidade larval no tamanho de adultos de Aedes aegypti criados em condições de laboratório. Revista da Sociedade Brasileira de Medicina Tropical 38: 64–66.
Gilchrist, B. M. & J. B. S. Haldane, 1947. Sex linkage and sex determination in a mosquito, Culex molestus. Hereditas 33(1–2): 175–190.
Graham, D. H., J. L. Holmes & W. C. Black, 2004. Identification of quantitative trait loci affecting sex determination in the eastern treehole mosquito (Ochlerotatus triseriatus). Journal of Heredity 95(1): 35–45.
Hickey, W. A. & G. B. Craig, 1966. Genetic distortion of sex ratio in a mosquito Aedes aegypti. Genetics 53(6): 1177–1196.
Hirst, A. G., D. Bonnet, D. V. P. Conway & T. Kiorboe, 2010. Does predation control adult sex ratios and longevities in marine pelagic copepods? Limnology & Oceanography 55(5): 2193–2206.
Koenraadt, C. J. M., 2008. Pupal dimensions as predictors of adult size in fitness studies of Aedes aegypti (Diptera: Culicidae). Journal of Medical Entomology 45(2): 331–336.
Lopez, L., E. Silva, M. Beltrão, R. Leandro, J. Barbosa & E. Beserra, 2011. Effect of tank bromeliad micro-environment on Aedes aegypti larval mortality. Hydrobiologia 665(1): 257–261.
Marten, G. G. & J. W. Reid, 2007. Cyclopoid copepods. Journal of the American Mosquitoes Control Association 23(2): 65–92.
Marten, G. G., R. Astaiza, M. F. Suarez, C. Monje & J. W. Reid, 1989. Natural control of larval Anopheles albimanus (Diptera: Culicidae) by the predator Mesocyclops (Copepoda: Cyclopoida). Journal of Medical Entomology 26(6): 624–627.
Marten, G. G., W. Che & E. S. Bordes, 1993. Compatibility of cyclopoid copepods with mosquito insecticides. Journal of the American Mosquito Control Association 9(2): 150–154.
Marten, G. G., E. S. Bordes & M. Nguyen, 1994. Use of cyclopoid copepods for mosquito control. Hydrobiologia 292–293(1): 491–496.
Marti, G. A., M. V. Micieli, A. C. Scorsetti & G. Liljesthröm, 2004. Evaluation of Mesocyclops annulatus (Copepoda: Cyclopoidea) as a control agent of Aedes aegypti (Diptera: Culicidae) in Argentina. Memórias do Instituto Oswaldo Cruz 99: 535–540.
Mercer, D. R., A. Schoergendorfer & R. Vandyke, 2008. Sexual differences in larval molting rates in a protandrous mosquito (Diptera: Culicidae) species, Aedes sierrensis. Journal of Medical Entomology 45(5): 861–866.
Mocellin, M. G., T. C. Simões, T. F. Nascimento, M. L. Teixeira, L. P. Lounibos & R. L. Oliveira, 2009. Bromeliad-inhabiting mosquitoes in an urban botanical garden of dengue endemic Rio de Janeiro – are bromeliads productive habitats for the invasive vectors Aedes aegypti and Aedes albopictus?. Memórias do Instituto Oswaldo Cruz 104(8): 1171–1176.
Nam, V. S., T. Y. Nguyen, B. H. Kay, G. G. Marten & J. W. Reid, 1998. Eradication of Aedes aegypti from a village in Vietnam, using copepods and community participation. The American Journal of Tropical Medicine and Hygiene 59(4): 657–660.
Nam, V. S., N. T. Yen, T. V. Phong, T. U. Ninh, L. Q. Mai, L. V. Lo, L. T. Nghia, A. Bektas, A. Briscombe, J. G. Aaskov, P. A. Ryan & B. H. Kay, 2005. Elimination of dengue by community programs using Mesocyclops (copepoda) against Aedes aegypti in central Vietnam. The American Journal of Tropical Medicine and Hygiene 72(1): 67–73.
Pamplona, L. d. G., C. H. Alencar, J. W. Lima & J. Heukelbach, 2009. Reduced oviposition of Aedes aegypti gravid females in domestic containers with predatory fish. Tropical Medicine & International Health 14(11): 1347–1350.
Panogadia-Reyes, C. M., E. I. C. Cruz & S. L. Bautista, 2004. Philippine species of Mesocyclops (Crustacea: Copepoda) as a biological control agent of Aedes aegypti (Linnaeus). Dengue Bulletin 28: 174–178.
Pernia, J., R. E. de Zoppi & M. Palacios-Caceres, 2007. Prey–predator relationship between the cyclopoids Mesocyclops longisetus and Mesocyclops meridianus with Anopheles aquasalis larvae. Journal of the American Mosquito Control Association 23(2): 166–171.
Quiroz-Martínez, H. & A. Rodríguez-Castro, 2007. Aquatic insects as predators of mosquito larvae. Journal of the American Mosquito Control Association 23(sp2): 110–117.
R Development Core Team, 2012. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. http://www.R-project.org/.
Rey, J. R., S. O’Connell, S. Suarez, Z. Menendez, L. P. Lounibos & G. Byer, 2004. Laboratory and field studies of Macrocyclops albidus (Crustacea: Copepoda) for biological control of mosquitoes in artificial containers in a subtropical environment. Journal of Vector Ecology 29(1): 124–134.
Serpa, L. L. N., S. D. C. B. Monteiro & J. C. Voltolini, 2008. Efeito da água de criação larval na oviposição de Aedes aegypti em laboratório. Revista da Sociedade Brasileira de Medicina Tropical 41: 515–517.
Statsoft, Inc., 2007. Statistica (data analysis software system). 8.0.
Sumanochitrapon, W., D. Strickman, R. Sithiprasasna, P. Kittayapong & B. L. Innis, 1998. Effect of size and geographic origin of Aedes aegypti on oral infection with dengue-2 virus. The American Journal of Tropical Medicine and Hygiene 58(3): 283–286.
Torres-Estrada, J. L., M. H. Rodríguez, L. Cruz-López & J. I. Arredondo-Jimenez, 2001. Selective oviposition by Aedes aegypti (Diptera: Culicidae) in response to Mesocyclops longisetus (Copepoda: Cyclopoidea) under laboratory and field conditions. Journal of Medical Entomology 38(2): 188–192.
Tranchida, M. C., M. V. Micieli, A. Maciá & J. J. García, 2009. Native Argentinean cyclopoids (Crustacea: Copepoda) as predators of Aedes aegypti and Culex pipiens (Diptera: Culicidae) mosquitoes. Revista de Biología Tropical 57: 1059–1068.
Vonesh, J. R. & L. Blaustein, 2010. Predator-induced shifts in mosquito oviposition site selection: a meta-analysis and implications for vector control. Israel Journal of Ecology & Evolution 56(3–4): 263–279.
Acknowledgments
This work is supported by a research fellowship from CAPES to HCBC and BQS. We would like to thank: E.L. Leite and M.L.V. Diniz.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Katya E. Kovalenko
Rights and permissions
About this article
Cite this article
Cardôso, H.C.B., da Silva, B.Q., de Assis, T.B. et al. Effects of predation by the copepod Mesocyclops ogunnus on the sex ratios of mosquito Aedes albopictus . Hydrobiologia 705, 55–61 (2013). https://doi.org/10.1007/s10750-012-1379-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-012-1379-3