Abstract
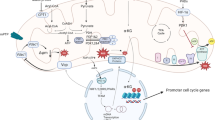
Mitochondrial dysfunction underlines a multitude of pathologies; however, studies are scarce that rescue the mitochondria for cellular resuscitation. Exploration into the protective role of mitochondrial transcription factor A (TFAM) and its mitochondrial functions respective to cardiomyocyte death are in need of further investigation. TFAM is a gene regulator that acts to mitigate calcium mishandling and ROS production by wrapping around mitochondrial DNA (mtDNA) complexes. TFAM’s regulatory functions over serca2a, NFAT, and Lon protease contribute to cardiomyocyte stability. Calcium- and ROS-dependent proteases, calpains, and matrix metalloproteinases (MMPs) are abundantly found upregulated in the failing heart. TFAM’s regulatory role over ROS production and calcium mishandling leads to further investigation into the cardioprotective role of exogenous TFAM. In an effort to restabilize physiological and contractile activity of cardiomyocytes in HF models, we propose that TFAM-packed exosomes (TFAM-PE) will act therapeutically by mitigating mitochondrial dysfunction. Notably, this is the first mention of exosomal delivery of transcription factors in the literature. Here we elucidate the role of TFAM in mitochondrial rescue and focus on its therapeutic potential.






Similar content being viewed by others
References
Ekstrand MI, Falkenberg M, Rantanen A, Park CB, Gaspari M, Hultenby K, Rustin P, Gustafsson CM, Larsson NG (2004) Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Hum Mol Genet 13(9):935–944. doi:10.1093/hmg/ddh109
Larsson NG, Wang J, Wilhelmsson H, Oldfors A, Rustin P, Lewandoski M, Barsh GS, Clayton DA (1998) Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet 18(3):231–236. doi:10.1038/ng0398-231
Alam TI, Kanki T, Muta T, Ukaji K, Abe Y, Nakayama H, Takio K, Hamasaki N, Kang D (2003) Human mitochondrial DNA is packaged with TFAM. Nucleic Acids Res 31(6):1640–1645
Virbasius JV, Scarpulla RC (1994) Activation of the human mitochondrial transcription factor A gene by nuclear respiratory factors: a potential regulatory link between nuclear and mitochondrial gene expression in organelle biogenesis. Proc Natl Acad Sci USA 91(4):1309–1313
Kukat C, Wurm CA, Spåhr H, Falkenberg M, Larsson N-G, Jakobs S (2011) Super-resolution microscopy reveals that mammalian mitochondrial nucleoids have a uniform size and frequently contain a single copy of mtDNA. Proc Natl Acad Sci USA 108(33):13534–13539. doi:10.1073/pnas.1109263108
Kukat C, Davies KM, Wurm CA, Spahr H, Bonekamp NA, Kuhl I, Joos F, Polosa PL, Park CB, Posse V, Falkenberg M, Jakobs S, Kuhlbrandt W, Larsson NG (2015) Cross-strand binding of TFAM to a single mtDNA molecule forms the mitochondrial nucleoid. Proc Natl Acad Sci USA. doi:10.1073/pnas.1512131112
Kienhofer J, Haussler DJ, Ruckelshausen F, Muessig E, Weber K, Pimentel D, Ullrich V, Burkle A, Bachschmid MM (2009) Association of mitochondrial antioxidant enzymes with mitochondrial DNA as integral nucleoid constituents. FASEB J 23(7):2034–2044. doi:10.1096/fj.08-113571
Hensen F, Cansiz S, Gerhold JM, Spelbrink JN (2014) To be or not to be a nucleoid protein: a comparison of mass-spectrometry based approaches in the identification of potential mtDNA-nucleoid associated proteins. Biochimie 100:219–226. doi:10.1016/j.biochi.2013.09.017
Falkenberg M, Larsson NG, Gustafsson CM (2007) DNA replication and transcription in mammalian mitochondria. Annu Rev Biochem 76:679–699. doi:10.1146/annurev.biochem.76.060305.152028
Falkenberg M, Gaspari M, Rantanen A, Trifunovic A, Larsson NG, Gustafsson CM (2002) Mitochondrial transcription factors B1 and B2 activate transcription of human mtDNA. Nat Genet 31(3):289–294. doi:10.1038/ng909
Seidel-Rogol BL, McCulloch V, Shadel GS (2003) Human mitochondrial transcription factor B1 methylates ribosomal RNA at a conserved stem-loop. Nat Genet 33(1):23–24. doi:10.1038/ng1064
Fisher RP, Lisowsky T, Parisi MA, Clayton DA (1992) DNA wrapping and bending by a mitochondrial high mobility group-like transcriptional activator protein. J Biol Chem 267(5):3358–3367
Correia RL, Oba-Shinjo SM, Uno M, Huang N, Marie SK (2011) Mitochondrial DNA depletion and its correlation with TFAM, TFB1M, TFB2 M and POLG in human diffusely infiltrating astrocytomas. Mitochondrion 11(1):48–53. doi:10.1016/j.mito.2010.07.001
Morozov YI, Agaronyan K, Cheung AC, Anikin M, Cramer P, Temiakov D (2014) A novel intermediate in transcription initiation by human mitochondrial RNA polymerase. Nucleic Acids Res 42(6):3884–3893. doi:10.1093/nar/gkt1356
Morozov YI, Parshin AV, Agaronyan K, Cheung AC, Anikin M, Cramer P, Temiakov D (2015) A model for transcription initiation in human mitochondria. Nucleic Acids Res 43(7):3726–3735. doi:10.1093/nar/gkv235
Surovtseva YV, Shadel GS (2013) Transcription-independent role for human mitochondrial RNA polymerase in mitochondrial ribosome biogenesis. Nucleic Acids Res 41(4):2479–2488. doi:10.1093/nar/gks1447
Cotney J, McKay SE, Shadel GS (2009) Elucidation of separate, but collaborative functions of the rRNA methyltransferase-related human mitochondrial transcription factors B1 and B2 in mitochondrial biogenesis reveals new insight into maternally inherited deafness. Hum Mol Genet 18(14):2670–2682. doi:10.1093/hmg/ddp208
Metodiev MD, Lesko N, Park CB, Camara Y, Shi Y, Wibom R, Hultenby K, Gustafsson CM, Larsson NG (2009) Methylation of 12S rRNA is necessary for in vivo stability of the small subunit of the mammalian mitochondrial ribosome. Cell Metab 9(4):386–397. doi:10.1016/j.cmet.2009.03.001
Wang J, Wilhelmsson H, Graff C, Li H, Oldfors A, Rustin P, Bruning JC, Kahn CR, Clayton DA, Barsh GS, Thoren P, Larsson NG (1999) Dilated cardiomyopathy and atrioventricular conduction blocks induced by heart-specific inactivation of mitochondrial DNA gene expression. Nat Genet 21(1):133–137. doi:10.1038/5089
Lauritzen KH, Kleppa L, Aronsen JM, Eide L, Carlsen H, Haugen OP, Sjaastad I, Klungland A, Rasmussen LJ, Attramadal H, Storm-Mathisen J, Bergersen LH (2015) Impaired dynamics and function of mitochondria caused by mtDNA toxicity leads to heart failure. Am J Physiol Heart Circ Physiol. doi:10.1152/ajpheart.00253.2014
Deocaris CC, Kaul SC, Wadhwa R (2006) On the brotherhood of the mitochondrial chaperones mortalin and heat shock protein 60. Cell Stress Chaperones 11(2):116–128
Kao TY, Chiu YC, Fang WC, Cheng CW, Kuo CY, Juan HF, Wu SH, Lee AY (2015) Mitochondrial Lon regulates apoptosis through the association with Hsp60-mtHsp70 complex. Cell Death Dis 6:e1642. doi:10.1038/cddis.2015.9
Santos JM, Mishra M, Kowluru RA (2014) Posttranslational modification of mitochondrial transcription factor A in impaired mitochondria biogenesis: implications in diabetic retinopathy and metabolic memory phenomenon. Exp Eye Res 121:168–177. doi:10.1016/j.exer.2014.02.010
Ciesielski GL, Plotka M, Manicki M, Schilke BA, Dutkiewicz R, Sahi C, Marszalek J, Craig EA (2013) Nucleoid localization of Hsp40 Mdj1 is important for its function in maintenance of mitochondrial DNA. Biochim Biophys Acta 1833(10):2233–2243. doi:10.1016/j.bbamcr.2013.05.012
Naka KK, Vezyraki P, Kalaitzakis A, Zerikiotis S, Michalis L, Angelidis C (2014) Hsp70 regulates the doxorubicin-mediated heart failure in Hsp70-transgenic mice. Cell Stress Chaperones 19(6):853–864. doi:10.1007/s12192-014-0509-4
Ko SK, Kim J, Na DC, Park S, Park SH, Hyun JY, Baek KH, Kim ND, Kim NK, Park YN, Song K, Shin I (2015) A small molecule inhibitor of ATPase activity of HSP70 induces apoptosis and has antitumor activities. Chem Biol 22(3):391–403. doi:10.1016/j.chembiol.2015.02.004
Xu T, Zhang B, Yang F, Cai C, Wang G, Han Q, Zou L (2015) HSF1 and NF-kappaB p65 participate in the process of exercise preconditioning attenuating pressure overload-induced pathological cardiac hypertrophy. Biochem Biophys Res Commun 460(3):622–627. doi:10.1016/j.bbrc.2015.03.079
Liu Q, Chen Y, Auger-Messier M, Molkentin JD (2012) Interaction between NFkappaB and NFAT coordinates cardiac hypertrophy and pathological remodeling. Circ Res 110(8):1077–1086. doi:10.1161/circresaha.111.260729
Givvimani S, Munjal C, Tyagi N, Sen U, Metreveli N, Tyagi SC (2012) Mitochondrial division/mitophagy inhibitor (Mdivi) ameliorates pressure overload induced heart failure. PLoS One 7(3):e32388. doi:10.1371/journal.pone.0032388
Givvimani S, Pushpakumar SB, Metreveli N, Veeranki S, Kundu S, Tyagi SC (2015) Role of mitochondrial fission and fusion in cardiomyocyte contractility. Int J Cardiol 187:325–333. doi:10.1016/j.ijcard.2015.03.352
Chen Y, Sparks M, Bhandari P, Matkovich SJ, Dorn GW 2nd (2014) Mitochondrial genome linearization is a causative factor for cardiomyopathy in mice and Drosophila. Antioxid Redox Signal 21(14):1949–1959. doi:10.1089/ars.2013.5432
Ishihara T, Ban-Ishihara R, Maeda M, Matsunaga Y, Ichimura A, Kyogoku S, Aoki H, Katada S, Nakada K, Nomura M, Mizushima N, Mihara K, Ishihara N (2015) Dynamics of mitochondrial DNA nucleoids regulated by mitochondrial fission is essential for maintenance of homogeneously active mitochondria during neonatal heart development. Mol Cell Biol 35(1):211–223. doi:10.1128/mcb.01054-14
Papanicolaou KN, Khairallah RJ, Ngoh GA, Chikando A, Luptak I, O’Shea KM, Riley DD, Lugus JJ, Colucci WS, Lederer WJ, Stanley WC, Walsh K (2011) Mitofusin-2 maintains mitochondrial structure and contributes to stress-induced permeability transition in cardiac myocytes. Mol Cell Biol 31(6):1309–1328. doi:10.1128/mcb.00911-10
Chen Y, Csordas G, Jowdy C, Schneider TG, Csordas N, Wang W, Liu Y, Kohlhaas M, Meiser M, Bergem S, Nerbonne JM, Dorn GW 2nd, Maack C (2012) Mitofusin 2-containing mitochondrial-reticular microdomains direct rapid cardiomyocyte bioenergetic responses via interorganelle Ca(2+) crosstalk. Circ Res 111(7):863–875. doi:10.1161/circresaha.112.266585
Li D, Li X, Guan Y, Guo X (2015) Mitofusin-2-mediated tethering of mitochondria and endoplasmic reticulum promotes cell cycle arrest of vascular smooth muscle cells in G0/G1 phase. Acta Biochim Biophys Sin 47(6):441–450. doi:10.1093/abbs/gmv035
Williams GS, Boyman L, Lederer WJ (2015) Mitochondrial calcium and the regulation of metabolism in the heart. J Mol Cell Cardiol 78:35–45. doi:10.1016/j.yjmcc.2014.10.019
Luongo TS, Lambert JP, Yuan A, Zhang X, Gross P, Song J, Shanmughapriya S, Gao E, Jain M, Houser SR, Koch WJ, Cheung JY, Madesh M, Elrod JW (2015) The mitochondrial calcium uniporter matches energetic supply with cardiac workload during stress and modulates permeability transition. Cell Rep 12(1):23–34. doi:10.1016/j.celrep.2015.06.017
Davidson SM, Foote K, Kunuthur S, Gosain R, Tan N, Tyser R, Zhao YJ, Graeff R, Ganesan A, Duchen MR, Patel S, Yellon DM (2015) Inhibition of NAADP signalling on reperfusion protects the heart by preventing lethal calcium oscillations via two-pore channel 1 and opening of the mitochondrial permeability transition pore. Cardiovasc Res. doi:10.1093/cvr/cvv226
Chitra L, Boopathy R (2014) Altered mitochondrial biogenesis and its fusion gene expression is involved in the high-altitude adaptation of rat lung. Respir Physiol Neurobiol 192:74–84. doi:10.1016/j.resp.2013.12.007
Cartoni R, Leger B, Hock MB, Praz M, Crettenand A, Pich S, Ziltener JL, Luthi F, Deriaz O, Zorzano A, Gobelet C, Kralli A, Russell AP (2005) Mitofusins 1/2 and ERRalpha expression are increased in human skeletal muscle after physical exercise. J Physiol 567(Pt 1):349–358. doi:10.1113/jphysiol.2005.092031
Karamanlidis G, Nascimben L, Couper GS, Shekar PS, del Monte F, Tian R (2010) Defective DNA replication impairs mitochondrial biogenesis in human failing hearts. Circ Res 106(9):1541–1548. doi:10.1161/circresaha.109.212753
Ikeda M, Ide T, Fujino T, Arai S, Saku K, Kakino T, Tyynismaa H, Yamasaki T, Yamada K, Kang D, Suomalainen A, Sunagawa K (2015) Overexpression of TFAM or twinkle increases mtDNA copy number and facilitates cardioprotection associated with limited mitochondrial oxidative stress. PLoS One 10(3):e0119687. doi:10.1371/journal.pone.0119687
Quinones-Lombrana A, Blanco JG (2015) Chromosome 21-derived hsa-miR-155-5p regulates mitochondrial biogenesis by targeting mitochondrial transcription factor A (TFAM). Biochim Biophys Acta 1852(7):1420–1427. doi:10.1016/j.bbadis.2015.04.004
Yamamoto H, Morino K, Nishio Y, Ugi S, Yoshizaki T, Kashiwagi A, Maegawa H (2012) MicroRNA-494 regulates mitochondrial biogenesis in skeletal muscle through mitochondrial transcription factor A and Forkhead box j3. Am J Physiol Endocrinol Metab 303(12):E1419–E1427. doi:10.1152/ajpendo.00097.2012
Li S, Yang G (2015) Hydrogen sulfide maintains mitochondrial DNA replication via demethylation of TFAM. Antioxid Redox Signal 23(7):630–642. doi:10.1089/ars.2014.6186
Zhang F, Qi Y, Zhou K, Zhang G, Linask K, Xu H (2015) The cAMP phosphodiesterase Prune localizes to the mitochondrial matrix and promotes mtDNA replication by stabilizing TFAM. EMBO Rep 16(4):520–527. doi:10.15252/embr.201439636
Chaturvedi P, Kalani A, Givvimani S, Kamat PK, Familtseva A, Tyagi SC (2014) Differential regulation of DNA methylation versus histone acetylation in cardiomyocytes during HHcy in vitro and in vivo: an epigenetic mechanism. Physiol Genomics 46(7):245–255. doi:10.1152/physiolgenomics.00168.2013
Kong X, Wang R, Xue Y, Liu X, Zhang H, Chen Y, Fang F, Chang Y (2010) Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS One 5(7):e11707. doi:10.1371/journal.pone.0011707
Zhang X, Ren X, Zhang Q, Li Z, Ma S, Bao J, Li Z, Bai X, Zheng L, Zhang Z, Shang S, Zhang C, Wang C, Cao L, Wang Q, Ji J (2015) PGC-1alpha/ERRalpha-Sirt3 pathway regulates DAergic neuronal death by directly deacetylating SOD2 and ATP synthase beta. Antioxid Redox Signal. doi:10.1089/ars.2015.6403
Uguccioni G, Hood DA (2011) The importance of PGC-1alpha in contractile activity-induced mitochondrial adaptations. Am J Physiol Endocrinol Metab 300(2):E361–E371. doi:10.1152/ajpendo.00292.2010
Kowluru RA, Santos JM, Zhong Q (2014) Sirt1, a negative regulator of matrix metalloproteinase-9 in diabetic retinopathy. Invest Ophthalmol Vis Sci 55(9):5653–5660. doi:10.1167/iovs.14-14383
Ferrari R, Cargnoni A, Curello S, Boffa GM, Ceconi C (1989) Effects of iloprost (ZK 36374) on glutathione status during ischaemia and reperfusion of rabbit isolated hearts. Br J Pharmacol 98(2):678–684
Loeper J, Goy J, Klein JM, Dufour M, Bedu O, Loeper S, Emerit J (1991) The evolution of oxidative stress indicators in the course of myocardial ischemia. Free Radic Res Commun 12–13(Pt 2):675–680
Loeper J, Goy J, Rozensztajn L, Bedu O, Moisson P (1991) Lipid peroxidation and protective enzymes during myocardial infarction. Clin Chim Acta Int J Clin Chem 196(2–3):119–125
Iqbal M, Cohen RI, Marzouk K, Liu SF (2002) Time course of nitric oxide, peroxynitrite, and antioxidants in the endotoxemic heart. Crit Care Med 30(6):1291–1296
Supinski GS, Murphy MP, Callahan LA (2009) MitoQ administration prevents endotoxin-induced cardiac dysfunction. Am J Physiol Regul Integr Comp Physiol 297(4):R1095–R1102. doi:10.1152/ajpregu.90902.2008
Laguens RP, Gomez-Dumm CL (1967) Fine structure of myocardial mitochondria in rats after exercise for one-half to two hours. Circ Res 21(3):271–279
Kane JJ, Murphy ML, Bissett JK, deSoyza N, Doherty JE, Straub KD (1975) Mitochondrial function, oxygen extraction, epicardial S-T segment changes and tritiated digoxin distribution after reperfusion of ischemic myocardium. Am J Cardiol 36(2):218–224
Jennings RB, Ganote CE (1976) Mitochondrial structure and function in acute myocardial ischemic injury. Circ Res 38(5 Suppl 1):I80–I91
Evans MJ, Scarpulla RC (1990) NRF-1: a trans-activator of nuclear-encoded respiratory genes in animal cells. Genes Dev 4(6):1023–1034
Chau CM, Evans MJ, Scarpulla RC (1992) Nuclear respiratory factor 1 activation sites in genes encoding the gamma-subunit of ATP synthase, eukaryotic initiation factor 2 alpha, and tyrosine aminotransferase. Specific interaction of purified NRF-1 with multiple target genes. J Biol Chem 267(10):6999–7006
Scarpulla RC (2002) Nuclear activators and coactivators in mammalian mitochondrial biogenesis. Biochim Biophys Acta 1576(1–2):1–14
Vercauteren K, Gleyzer N, Scarpulla RC (2008) PGC-1-related coactivator complexes with HCF-1 and NRF-2beta in mediating NRF-2(GABP)-dependent respiratory gene expression. J Biol Chem 283(18):12102–12111. doi:10.1074/jbc.M710150200
Gleyzer N, Vercauteren K, Scarpulla RC (2005) Control of mitochondrial transcription specificity factors (TFB1M and TFB2M) by nuclear respiratory factors (NRF-1 and NRF-2) and PGC-1 family coactivators. Mol Cell Biol 25(4):1354–1366. doi:10.1128/MCB.25.4.1354-1366.2005
Hickson-Bick DL, Jones C, Buja LM (2008) Stimulation of mitochondrial biogenesis and autophagy by lipopolysaccharide in the neonatal rat cardiomyocyte protects against programmed cell death. J Mol Cell Cardiol 44(2):411–418. doi:10.1016/j.yjmcc.2007.10.013
Suliman HB, Welty-Wolf KE, Carraway M, Tatro L, Piantadosi CA (2004) Lipopolysaccharide induces oxidative cardiac mitochondrial damage and biogenesis. Cardiovasc Res 64(2):279–288. doi:10.1016/j.cardiores.2004.07.005
Yue R, Xia X, Jiang J, Yang D, Han Y, Chen X, Cai Y, Li L, Wang WE, Zeng C (2015) Mitochondrial DNA oxidative damage contributes to cardiomyocyte ischemia/reperfusion-injury in rats: cardioprotective role of lycopene. J Cell Physiol. doi:10.1002/jcp.24941
Spinale FG, Coker ML, Thomas CV, Walker JD, Mukherjee R, Hebbar L (1998) Time-dependent changes in matrix metalloproteinase activity and expression during the progression of congestive heart failure: relation to ventricular and myocyte function. Circ Res 82(4):482–495
Siwik DA, Tzortzis JD, Pimental DR, Chang DL, Pagano PJ, Singh K, Sawyer DB, Colucci WS (1999) Inhibition of copper-zinc superoxide dismutase induces cell growth, hypertrophic phenotype, and apoptosis in neonatal rat cardiac myocytes in vitro. Circ Res 85(2):147–153
Vu TH, Werb Z (2000) Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev 14(17):2123–2133
Tyagi SC (1998) Dynamic role of extracellular matrix metalloproteinases in heart failure. Cardiovasc Pathol Off J Soc Cardiovasc Pathol 7(3):153–159
Weber KT, Sun Y, Tyagi SC, Cleutjens JP (1994) Collagen network of the myocardium: function, structural remodeling and regulatory mechanisms. J Mol Cell Cardiol 26(3):279–292. doi:10.1006/jmcc.1994.1036
Mujumdar VS, Smiley LM, Tyagi SC (2001) Activation of matrix metalloproteinase dilates and decreases cardiac tensile strength. Int J Cardiol 79(2–3):277–286
Lebrecht D, Setzer B, Ketelsen UP, Haberstroh J, Walker UA (2003) Time-dependent and tissue-specific accumulation of mtDNA and respiratory chain defects in chronic doxorubicin cardiomyopathy. Circulation 108(19):2423–2429. doi:10.1161/01.CIR.0000093196.59829.DF
Ide T, Tsutsui H, Hayashidani S, Kang D, Suematsu N, Nakamura K, Utsumi H, Hamasaki N, Takeshita A (2001) Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ Res 88(5):529–535
Kanazawa A, Nishio Y, Kashiwagi A, Inagaki H, Kikkawa R, Horiike K (2002) Reduced activity of mtTFA decreases the transcription in mitochondria isolated from diabetic rat heart. Am J Physiol Endocrinol Metab 282(4):E778–E785. doi:10.1152/ajpendo.00255.2001
Garnier A, Fortin D, Delomenie C, Momken I, Veksler V, Ventura-Clapier R (2003) Depressed mitochondrial transcription factors and oxidative capacity in rat failing cardiac and skeletal muscles. J Physiol 551(Pt 2):491–501. doi:10.1113/jphysiol.2003.045104
Ikeuchi M, Matsusaka H, Kang D, Matsushima S, Ide T, Kubota T, Fujiwara T, Hamasaki N, Takeshita A, Sunagawa K, Tsutsui H (2005) Overexpression of mitochondrial transcription factor a ameliorates mitochondrial deficiencies and cardiac failure after myocardial infarction. Circulation 112(5):683–690. doi:10.1161/CIRCULATIONAHA.104.524835
Russell LK, Mansfield CM, Lehman JJ, Kovacs A, Courtois M, Saffitz JE, Medeiros DM, Valencik ML, McDonald JA, Kelly DP (2004) Cardiac-specific induction of the transcriptional coactivator peroxisome proliferator-activated receptor gamma coactivator-1alpha promotes mitochondrial biogenesis and reversible cardiomyopathy in a developmental stage-dependent manner. Circ Res 94(4):525–533. doi:10.1161/01.RES.0000117088.36577.EB
Kunkel GH, Chaturvedi P, Tyagi SC (2015) Resuscitation of a dead cardiomyocyte. Heart Fail Rev 20(6):709–719. doi:10.1007/s10741-015-9501-z
Lu B, Lee J, Nie X, Li M, Morozov YI, Venkatesh S, Bogenhagen DF, Temiakov D, Suzuki CK (2013) Phosphorylation of human TFAM in mitochondria impairs DNA binding and promotes degradation by the AAA + Lon protease. Mol Cell 49(1):121–132. doi:10.1016/j.molcel.2012.10.023
Matsushima Y, Goto Y, Kaguni LS (2010) Mitochondrial Lon protease regulates mitochondrial DNA copy number and transcription by selective degradation of mitochondrial transcription factor A (TFAM). Proc Natl Acad Sci USA 107(43):18410–18415. doi:10.1073/pnas.1008924107
Fu GK, Markovitz DM (1998) The human LON protease binds to mitochondrial promoters in a single-stranded, site-specific, strand-specific manner. Biochemistry 37(7):1905–1909. doi:10.1021/bi970928c
Lu B, Yadav S, Shah PG, Liu T, Tian B, Pukszta S, Villaluna N, Kutejova E, Newlon CS, Santos JH, Suzuki CK (2007) Roles for the human ATP-dependent Lon protease in mitochondrial DNA maintenance. J Biol Chem 282(24):17363–17374. doi:10.1074/jbc.M611540200
Pinti M, Gibellini L, De Biasi S, Nasi M, Roat E, O’Connor JE, Cossarizza A (2011) Functional characterization of the promoter of the human Lon protease gene. Mitochondrion 11(1):200–206. doi:10.1016/j.mito.2010.09.010
Ngo JK, Davies KJ (2009) Mitochondrial Lon protease is a human stress protein. Free Radic Biol Med 46(8):1042–1048. doi:10.1016/j.freeradbiomed.2008.12.024
Kuo CY, Chiu YC, Lee AY, Hwang TL (2015) Mitochondrial Lon protease controls ROS-dependent apoptosis in cardiomyocyte under hypoxia. Mitochondrion 23:7–16. doi:10.1016/j.mito.2015.04.004
Li F, Wang Y, Zeller KI, Potter JJ, Wonsey DR, O’Donnell KA, Kim JW, Yustein JT, Lee LA, Dang CV (2005) Myc stimulates nuclearly encoded mitochondrial genes and mitochondrial biogenesis. Mol Cell Biol 25(14):6225–6234. doi:10.1128/mcb.25.14.6225-6234.2005
Mendelsohn AR, Larrick JW (2014) Partial reversal of skeletal muscle aging by restoration of normal NAD(+) levels. Rejuvenation Res 17(1):62–69. doi:10.1089/rej.2014.1546
Hwang HJ, Lynn SG, Vengellur A, Saini Y, Grier EA, Ferguson-Miller SM, LaPres JJ (2015) Hypoxia inducible factors modulate mitochondrial oxygen consumption and transcriptional regulation of nuclear-encoded electron transport chain genes. Biochemistry 54(24):3739–3748. doi:10.1021/bi5012892
Huttemann M, Klewer S, Lee I, Pecinova A, Pecina P, Liu J, Lee M, Doan JW, Larson D, Slack E, Maghsoodi B, Erickson RP, Grossman LI (2012) Mice deleted for heart-type cytochrome c oxidase subunit 7a1 develop dilated cardiomyopathy. Mitochondrion 12(2):294–304. doi:10.1016/j.mito.2011.11.002
Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL (2007) HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell 129(1):111–122. doi:10.1016/j.cell.2007.01.047
Vandewalle A, Tourneur E, Bens M, Chassin C, Werts C (2014) Calcineurin/NFAT signaling and innate host defence: a role for NOD1-mediated phagocytic functions. Cell Commun Signal 12:8. doi:10.1186/1478-811x-12-8
Minematsu H, Shin MJ, Celil Aydemir AB, Kim KO, Nizami SA, Chung GJ, Lee FY (2011) Nuclear presence of nuclear factor of activated T cells (NFAT) c3 and c4 is required for Toll-like receptor-activated innate inflammatory response of monocytes/macrophages. Cell Signal 23(11):1785–1793. doi:10.1016/j.cellsig.2011.06.013
Ma B, Yu J, Xie C, Sun L, Lin S, Ding J, Luo J, Cai H (2015) Toll-like receptors promote mitochondrial translocation of nuclear transcription factor nuclear factor of activated T-cells in prolonged microglial activation. J Neurosci 35(30):10799–10814. doi:10.1523/jneurosci.2455-14.2015
Fujino T, Ide T, Yoshida M, Onitsuka K, Tanaka A, Hata Y, Nishida M, Takehara T, Kanemaru T, Kitajima N, Takazaki S, Kurose H, Kang D, Sunagawa K (2012) Recombinant mitochondrial transcription factor A protein inhibits nuclear factor of activated T cells signaling and attenuates pathological hypertrophy of cardiac myocytes. Mitochondrion 12(4):449–458. doi:10.1016/j.mito.2012.06.002
Kim SC, Stice JP, Chen L, Jung JS, Gupta S, Wang Y, Baumgarten G, Trial J, Knowlton AA (2009) Extracellular heat shock protein 60, cardiac myocytes, and apoptosis. Circ Res 105(12):1186–1195. doi:10.1161/circresaha.109.209643
Li Y, Si R, Feng Y, Chen HH, Zou L, Wang E, Zhang M, Warren HS, Sosnovik DE, Chao W (2011) Myocardial ischemia activates an injurious innate immune signaling via cardiac heat shock protein 60 and Toll-like receptor 4. J Biol Chem 286(36):31308–31319. doi:10.1074/jbc.M111.246124
Dasu MR, Jialal I (2011) Free fatty acids in the presence of high glucose amplify monocyte inflammation via Toll-like receptors. Am J Physiol Endocrinol Metab 300(1):E145–E154. doi:10.1152/ajpendo.00490.2010
Wei M, Li Z, Xiao L, Yang Z (2015) Effects of ROS-relative NF-kappaB signaling on high glucose-induced TLR4 and MCP-1 expression in podocyte injury. Mol Immunol. doi:10.1016/j.molimm.2015.09.002
Williams CR, Gooch JL (2014) Calcineurin Abeta regulates NADPH oxidase (Nox) expression and activity via nuclear factor of activated T cells (NFAT) in response to high glucose. J Biol Chem 289(8):4896–4905. doi:10.1074/jbc.M113.514869
Sorescu D, Griendling KK (2002) Reactive oxygen species, mitochondria, and NAD(P)H oxidases in the development and progression of heart failure. Congest Heart Fail (Greenwich, Conn) 8(3):132–140
Sciarretta S, Yee D, Ammann P, Nagarajan N, Volpe M, Frati G, Sadoshima J (2015) Role of NADPH oxidase in the regulation of autophagy in cardiomyocytes. Clin Sci (London, England: 1979) 128(7):387–403. doi:10.1042/cs20140336
Chang H, Sheng JJ, Zhang L, Yue ZJ, Jiao B, Li JS, Yu ZB (2015) ROS-induced nuclear translocation of calpain-2 facilitates cardiomyocyte apoptosis in tail-suspended rats. J Cell Biochem. doi:10.1002/jcb.25176
Kang D, Kim SH, Hamasaki N (2007) Mitochondrial transcription factor A (TFAM): roles in maintenance of mtDNA and cellular functions. Mitochondrion 7(1–2):39–44. doi:10.1016/j.mito.2006.11.017
Banfi B, Molnar G, Maturana A, Steger K, Hegedus B, Demaurex N, Krause KH (2001) A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J Biol Chem 276(40):37594–37601. doi:10.1074/jbc.M103034200
Jagnandan D, Church JE, Banfi B, Stuehr DJ, Marrero MB, Fulton DJ (2007) Novel mechanism of activation of NADPH oxidase 5. calcium sensitization via phosphorylation. J Biol Chem 282(9):6494–6507. doi:10.1074/jbc.M608966200
Smith MA, Schnellmann RG (2012) Mitochondrial calpain 10 is degraded by Lon protease after oxidant injury. Arch Biochem Biophys 517(2):144–152. doi:10.1016/j.abb.2011.11.023
Rezende F, Lowe O, Helfinger V, Prior KK, Walter M, Zukunft S, Fleming I, Weissmann N, Brandes RP, Schroder K (2015) Unchanged NADPH oxidase activity in Nox1-Nox2-Nox4 triple knockout mice: what do NADPH-stimulated chemiluminescence assays really detect? Antioxid Redox Signal. doi:10.1089/ars.2015.6314
Kunkel GH, Chaturvedi P, Tyagi SC (2015) Resuscitation of a dead cardiomyocyte. Heart Fail Rev. doi:10.1007/s10741-015-9501-z
Wang Y, Tsui H, Ke Y, Shi Y, Li Y, Davies L, Cartwright EJ, Venetucci L, Zhang H, Terrar DA, Huang CL, Solaro RJ, Wang X, Lei M (2014) Pak1 is required to maintain ventricular Ca(2)(+) homeostasis and electrophysiological stability through SERCA2a regulation in mice. Circ Arrhythm Electrophysiol 7(5):938–948. doi:10.1161/circep.113.001198
Ke Y, Lei M, Collins TP, Rakovic S, Mattick PA, Yamasaki M, Brodie MS, Terrar DA, Solaro RJ (2007) Regulation of L-type calcium channel and delayed rectifier potassium channel activity by p21-activated kinase-1 in guinea pig sinoatrial node pacemaker cells. Circ Res 100(9):1317–1327. doi:10.1161/01.RES.0000266742.51389.a4
Huang H, Joseph LC, Gurin MI, Thorp EB, Morrow JP (2014) Extracellular signal-regulated kinase activation during cardiac hypertrophy reduces sarcoplasmic/endoplasmic reticulum calcium ATPase 2 (SERCA2) transcription. J Mol Cell Cardiol 75:58–63. doi:10.1016/j.yjmcc.2014.06.018
Prasad AM, Inesi G (2012) Regulation and rate limiting mechanisms of Ca2+ ATPase (SERCA2) expression in cardiac myocytes. Mol Cell Biochem 361(1–2):85–96. doi:10.1007/s11010-011-1092-y
Heinis FI, Andersson KB, Christensen G, Metzger JM (2013) Prominent heart organ-level performance deficits in a genetic model of targeted severe and progressive SERCA2 deficiency. PLoS One 8(11):e79609. doi:10.1371/journal.pone.0079609
Li L, Louch WE, Niederer SA, Andersson KB, Christensen G, Sejersted OM, Smith NP (2011) Calcium dynamics in the ventricular myocytes of SERCA2 knockout mice: a modeling study. Biophys J 100(2):322–331. doi:10.1016/j.bpj.2010.11.048
Stokke MK, Hougen K, Sjaastad I, Louch WE, Briston SJ, Enger UH, Andersson KB, Christensen G, Eisner DA, Sejersted OM, Trafford AW (2010) Reduced SERCA2 abundance decreases the propensity for Ca2+ wave development in ventricular myocytes. Cardiovasc Res 86(1):63–71. doi:10.1093/cvr/cvp401
Cutler MJ, Wan X, Laurita KR, Hajjar RJ, Rosenbaum DS (2009) Targeted SERCA2a gene expression identifies molecular mechanism and therapeutic target for arrhythmogenic cardiac alternans. Circ Arrhythm Electrophysiol 2(6):686–694. doi:10.1161/circep.109.863118
Hillestad V, Kramer F, Golz S, Knorr A, Andersson KB (1985) Christensen G (2013) Long-term levosimendan treatment improves systolic function and myocardial relaxation in mice with cardiomyocyte-specific disruption of the Serca2 gene. J Appl Physiol 115(10):1572–1580. doi:10.1152/japplphysiol.01044.2012
Swift F, Franzini-Armstrong C, Oyehaug L, Enger UH, Andersson KB, Christensen G, Sejersted OM, Louch WE (2012) Extreme sarcoplasmic reticulum volume loss and compensatory T-tubule remodeling after Serca2 knockout. Proc Natl Acad Sci USA 109(10):3997–4001. doi:10.1073/pnas.1120172109
Lu YM, Huang J, Shioda N, Fukunaga K, Shirasaki Y, Li XM, Han F (2011) CaMKIIdeltaB mediates aberrant NCX1 expression and the imbalance of NCX1/SERCA in transverse aortic constriction-induced failing heart. PLoS One 6(9):e24724. doi:10.1371/journal.pone.0024724
Stokke MK, Briston SJ, Jolle GF, Manzoor I, Louch WE, Oyehaug L, Christensen G, Eisner DA, Trafford AW, Sejersted OM, Sjaastad I (2011) Ca(2+) wave probability is determined by the balance between SERCA2-dependent Ca(2+) reuptake and threshold SR Ca(2+) content. Cardiovasc Res 90(3):503–512. doi:10.1093/cvr/cvr013
Sikkel MB, Hayward C, MacLeod KT, Harding SE, Lyon AR (2014) SERCA2a gene therapy in heart failure: an anti-arrhythmic positive inotrope. Br J Pharmacol 171(1):38–54. doi:10.1111/bph.12472
Lipskaia L, Chemaly ER, Hadri L, Lompre AM, Hajjar RJ (2010) Sarcoplasmic reticulum Ca(2+) ATPase as a therapeutic target for heart failure. Expert Opin Biol Ther 10(1):29–41. doi:10.1517/14712590903321462
Park WJ, Oh JG (2013) SERCA2a: a prime target for modulation of cardiac contractility during heart failure. BMB Rep 46(5):237–243
Cutler MJ, Wan X, Plummer BN, Liu H, Deschenes I, Laurita KR, Hajjar RJ, Rosenbaum DS (2012) Targeted sarcoplasmic reticulum Ca2+ ATPase 2a gene delivery to restore electrical stability in the failing heart. Circulation 126(17):2095–2104. doi:10.1161/circulationaha.111.071480
Lyon AR, Bannister ML, Collins T, Pearce E, Sepehripour AH, Dubb SS, Garcia E, O’Gara P, Liang L, Kohlbrenner E, Hajjar RJ, Peters NS, Poole-Wilson PA, Macleod KT, Harding SE (2011) SERCA2a gene transfer decreases sarcoplasmic reticulum calcium leak and reduces ventricular arrhythmias in a model of chronic heart failure. Circ Arrhythm Electrophysiol 4(3):362–372. doi:10.1161/circep.110.961615
Xin W, Li X, Lu X, Niu K, Cai J (2011) Improved cardiac function after sarcoplasmic reticulum Ca(2+)-ATPase gene transfer in a heart failure model induced by chronic myocardial ischaemia. Acta Cardiol 66(1):57–64
Zsebo K, Yaroshinsky A, Rudy JJ, Wagner K, Greenberg B, Jessup M, Hajjar RJ (2014) Long-term effects of AAV1/SERCA2a gene transfer in patients with severe heart failure: analysis of recurrent cardiovascular events and mortality. Circ Res 114(1):101–108. doi:10.1161/circresaha.113.302421
Hayward C, Banner NR, Morley-Smith A, Lyon AR, Harding SE (2015) The current and future landscape of SERCA gene therapy for heart failure: a clinical perspective. Hum Gene Ther 26(5):293–304. doi:10.1089/hum.2015.018
Watanabe A, Arai M, Koitabashi N, Niwano K, Ohyama Y, Yamada Y, Kato N, Kurabayashi M (2011) Mitochondrial transcription factors TFAM and TFB2M regulate Serca2 gene transcription. Cardiovasc Res 90(1):57–67. doi:10.1093/cvr/cvq374
Takahashi M, Tanonaka K, Yoshida H, Koshimizu M, Daicho T, Oikawa R, Takeo S (2006) Possible involvement of calpain activation in pathogenesis of chronic heart failure after acute myocardial infarction. J Cardiovasc Pharmacol 47(3):413–421. doi:10.1097/01.fjc.0000210074.56614.3b
Undrovinas A, Maltsev VA, Sabbah HN (2013) Calpain inhibition reduces amplitude and accelerates decay of the late sodium current in ventricular myocytes from dogs with chronic heart failure. PLoS One 8(4):e54436. doi:10.1371/journal.pone.0054436
Shintani-Ishida K, Yoshida K (2015) Mitochondrial m-calpain opens the mitochondrial permeability transition pore in ischemia-reperfusion. Int J Cardiol 197:26–32. doi:10.1016/j.ijcard.2015.06.010
Orrenius S, Gogvadze V, Zhivotovsky B (2015) Calcium and mitochondria in the regulation of cell death. Biochem Biophys Res Commun 460(1):72–81. doi:10.1016/j.bbrc.2015.01.137
Bhosale G, Sharpe JA, Sundier SY, Duchen MR (2015) Calcium signaling as a mediator of cell energy demand and a trigger to cell death. Ann N Y Acad Sci 1350(1):107–116. doi:10.1111/nyas.12885
Griffiths EJ, Balaska D, Cheng WH (2010) The ups and downs of mitochondrial calcium signalling in the heart. Biochim Biophys Acta 1797(6–7):856–864. doi:10.1016/j.bbabio.2010.02.022
Cao G, Xing J, Xiao X, Liou AK, Gao Y, Yin XM, Clark RS, Graham SH, Chen J (2007) Critical role of calpain I in mitochondrial release of apoptosis-inducing factor in ischemic neuronal injury. J Neurosci 27(35):9278–9293. doi:10.1523/jneurosci.2826-07.2007
Moshal KS, Singh M, Sen U, Rosenberger DS, Henderson B, Tyagi N, Zhang H, Tyagi SC (2006) Homocysteine-mediated activation and mitochondrial translocation of calpain regulates MMP-9 in MVEC. Am J Physiol Heart Circ Physiol 291(6):H2825–H2835. doi:10.1152/ajpheart.00377.2006
Chen M, He H, Zhan S, Krajewski S, Reed JC, Gottlieb RA (2001) Bid is cleaved by calpain to an active fragment in vitro and during myocardial ischemia/reperfusion. J Biol Chem 276(33):30724–30728. doi:10.1074/jbc.M103701200
Thomas RR, Khan SM, Smigrodzki RM, Onyango IG, Dennis J, Khan OM, Portelli FR, Bennett JP Jr (2012) RhTFAM treatment stimulates mitochondrial oxidative metabolism and improves memory in aged mice. Aging (Albany NY) 4(9):620–635
Chaturvedi P, Kalani A, Medina I, Familtseva A, Tyagi SC (2015) Cardiosome mediated regulation of MMP9 in diabetic heart: role of mir29b and mir455 in exercise. J Cell Mol Med. doi:10.1111/jcmm.12589
Mishra PK, Chavali V, Metreveli N, Tyagi SC (2012) Ablation of MMP9 induces survival and differentiation of cardiac stem cells into cardiomyocytes in the heart of diabetics: a role of extracellular matrix. Can J Physiol Pharmacol 90(3):353–360. doi:10.1139/y11-131
Chaturvedi P, Kalani A, Familtseva A, Kamat PK, Metreveli N, Tyagi SC (2015) Cardiac tissue inhibitor of matrix metalloprotease 4 dictates cardiomyocyte contractility and differentiation of embryonic stem cells into cardiomyocytes: road to therapy. Int J Cardiol 184C:350–363. doi:10.1016/j.ijcard.2015.01.091
Wang Y, Zhang L, Li Y, Chen L, Wang X, Guo W, Zhang X, Qin G, He SH, Zimmerman A, Liu Y, Kim IM, Weintraub NL, Tang Y (2015) Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int J Cardiol 192:61–69. doi:10.1016/j.ijcard.2015.05.020
Kalani A, Kamat PK, Chaturvedi P, Tyagi SC, Tyagi N (2014) Curcumin-primed exosomes mitigate endothelial cell dysfunction during hyperhomocysteinemia. Life Sci 107(1–2):1–7. doi:10.1016/j.lfs.2014.04.018
Chen L, Wang Y, Pan Y, Zhang L, Shen C, Qin G, Ashraf M, Weintraub N, Ma G, Tang Y (2013) Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochem Biophys Res Commun 431(3):566–571. doi:10.1016/j.bbrc.2013.01.015
Vicencio JM, Yellon DM, Sivaraman V, Das D, Boi-Doku C, Arjun S, Zheng Y, Riquelme JA, Kearney J, Sharma V, Multhoff G, Hall AR, Davidson SM (2015) Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J Am Coll Cardiol 65(15):1525–1536. doi:10.1016/j.jacc.2015.02.026
Das S, Halushka MK (2015) Extracellular vesicle microRNA transfer in cardiovascular disease. Cardiovasc Pathol Off J Soc Cardiovasc Pathol 24(4):199–206. doi:10.1016/j.carpath.2015.04.007
Gray WD, French KM, Ghosh-Choudhary S, Maxwell JT, Brown ME, Platt MO, Searles CD, Davis ME (2015) Identification of therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circ Res 116(2):255–263. doi:10.1161/circresaha.116.304360
Chaturvedi P, Kalani A, Medina I, Familtseva A, Tyagi SC (2015) Cardiosome mediated regulation of MMP9 in diabetic heart: role of mir29b and mir455 in exercise. J Cell Mol Med 19(9):2153–2161. doi:10.1111/jcmm.12589
Ong SG, Lee WH, Huang M, Dey D, Kodo K, Sanchez-Freire V, Gold JD, Wu JC (2014) Cross talk of combined gene and cell therapy in ischemic heart disease: role of exosomal microRNA transfer. Circulation 130(11 Suppl 1):S60–S69. doi:10.1161/circulationaha.113.007917
Cai X, Bao L, Ren J, Li Y, Zhang Z (2016) Grape seed procyanidin B2 protects podocytes from high glucose-induced mitochondrial dysfunction and apoptosis via the AMPK-SIRT1-PGC-1alpha axis in vitro. Food Funct 7(2):805–815. doi:10.1039/c5fo01062d
Yoshino M, Naka A, Sakamoto Y, Shibasaki A, Toh M, Tsukamoto S, Kondo K, Iida K (2015) Dietary isoflavone daidzein promotes Tfam expression that increases mitochondrial biogenesis in C2C12 muscle cells. J Nutr Biochem 26(11):1193–1199. doi:10.1016/j.jnutbio.2015.05.010
Qin G, Wu M, Wang J, Xu Z, Xia J, Sang N (2016) Sulfur dioxide contributes to the cardiac and mitochondrial dysfunction in rats. Toxicol Sci Off J Soc Toxicol. doi:10.1093/toxsci/kfw048
Sreekumar PG, Ishikawa K, Spee C, Mehta HH, Wan J, Yen K, Cohen P, Kannan R, Hinton DR (2016) The mitochondrial-derived peptide humanin protects RPE cells from oxidative stress, senescence, and mitochondrial dysfunction. Invest Ophthalmol Vis Sci 57(3):1238–1253. doi:10.1167/iovs.15-17053
Pillai VB, Samant S, Sundaresan NR, Raghuraman H, Kim G, Bonner MY, Arbiser JL, Walker DI, Jones DP, Gius D, Gupta MP (2015) Honokiol blocks and reverses cardiac hypertrophy in mice by activating mitochondrial Sirt3. Nat Commun 6:6656. doi:10.1038/ncomms7656
Mei H, Sun S, Bai Y, Chen Y, Chai R, Li H (2015) Reduced mtDNA copy number increases the sensitivity of tumor cells to chemotherapeutic drugs. Cell Death Dis 6:e1710. doi:10.1038/cddis.2015.78
Chandrasekaran K, Anjaneyulu M, Inoue T, Choi J, Sagi AR, Chen C, Ide T, Russell JW (2015) Mitochondrial transcription factor A regulation of mitochondrial degeneration in experimental diabetic neuropathy. Am J Physiol Endocrinol Metab 309(2):E132–E141. doi:10.1152/ajpendo.00620.2014
Hayashi Y, Yoshida M, Yamato M, Ide T, Wu Z, Ochi-Shindou M, Kanki T, Kang D, Sunagawa K, Tsutsui H, Nakanishi H (2008) Reverse of age-dependent memory impairment and mitochondrial DNA damage in microglia by an overexpression of human mitochondrial transcription factor a in mice. J Neurosci 28(34):8624–8634. doi:10.1523/jneurosci.1957-08.2008
Zhang Q, Yu JT, Wang P, Chen W, Wu ZC, Jiang H, Tan L (2011) Mitochondrial transcription factor A (TFAM) polymorphisms and risk of late-onset Alzheimer’s disease in Han Chinese. Brain Res 1368:355–360. doi:10.1016/j.brainres.2010.10.074
Aguirre-Rueda D, Guerra-Ojeda S, Aldasoro M, Iradi A, Obrador E, Ortega A, Mauricio MD, Vila JM, Valles SL (2015) Astrocytes protect neurons from Abeta1-42 peptide-induced neurotoxicity increasing TFAM and PGC-1 and decreasing PPAR-gamma and SIRT-1. Int J Med Sci 12(1):48–56. doi:10.7150/ijms.10035
Xu S, Zhong M, Zhang L, Wang Y, Zhou Z, Hao Y, Zhang W, Yang X, Wei A, Pei L, Yu Z (2009) Overexpression of Tfam protects mitochondria against beta-amyloid-induced oxidative damage in SH-SY5Y cells. FEBS J 276(14):3800–3809. doi:10.1111/j.1742-4658.2009.07094.x
Diez JJ, Iglesias P (2003) The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur J Endocrinol 148(3):293–300
Duan J, Yin Y, Cui J, Yan J, Zhu Y, Guan Y, Wei G, Weng Y, Wu X, Guo C, Wang Y, Xi M, Wen A (2015) Chikusetsu saponin IVa ameliorates cerebral ischemia reperfusion injury in diabetic mice via adiponectin-mediated AMPK/GSK-3beta pathway in vivo and in vitro. Mol Neurobiol. doi:10.1007/s12035-014-9033-x
Yan W, Zhang F, Zhang R, Zhang X, Wang Y, Zhou F, Xia Y, Liu P, Gao C, Wang H, Zhang L, Zhou J, Gao F, Gao E, Koch WJ, Wang H, Cheng H, Qu Y, Tao L (2014) Adiponectin regulates SR Ca(2+) cycling following ischemia/reperfusion via sphingosine 1-phosphate-CaMKII signaling in mice. J Mol Cell Cardiol 74:183–192. doi:10.1016/j.yjmcc.2014.05.010
Wang KZ, Zhu J, Dagda RK, Uechi G, Cherra SJ 3rd, Gusdon AM, Balasubramani M, Chu CT (2014) ERK-mediated phosphorylation of TFAM downregulates mitochondrial transcription: implications for Parkinson’s disease. Mitochondrion 17:132–140. doi:10.1016/j.mito.2014.04.008
Santos JM, Kowluru RA (2011) Role of mitochondria biogenesis in the metabolic memory associated with the continued progression of diabetic retinopathy and its regulation by lipoic acid. Invest Ophthalmol Vis Sci 52(12):8791–8798. doi:10.1167/iovs.11-8203
He B, Meng YH, Mivechi NF (1998) Glycogen synthase kinase 3beta and extracellular signal-regulated kinase inactivate heat shock transcription factor 1 by facilitating the disappearance of transcriptionally active granules after heat shock. Mol Cell Biol 18(11):6624–6633
Ngamsiri P, Watcharasit P, Satayavivad J (2014) Glycogen synthase kinase-3 (GSK3) controls deoxyglucose-induced mitochondrial biogenesis in human neuroblastoma SH-SY5Y cells. Mitochondrion 14(1):54–63. doi:10.1016/j.mito.2013.11.003
Sunaga D, Tanno M, Kuno A, Ishikawa S, Ogasawara M, Yano T, Miki T, Miura T (2014) Accelerated recovery of mitochondrial membrane potential by GSK-3beta inactivation affords cardiomyocytes protection from oxidant-induced necrosis. PLoS One 9(11):e112529. doi:10.1371/journal.pone.0112529
Acknowledgments
The study was supported by NIH Grants HL-74185 and HL-108621, AHA Grant 15POST23110021 to PC and NIH F31 Grant 1F31HL132527-01 to GHK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
George H. Kunkel, Pankaj Chaturvedi and Suresh C. Tyagi have claimed that there are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Kunkel, G.H., Chaturvedi, P. & Tyagi, S.C. Mitochondrial pathways to cardiac recovery: TFAM. Heart Fail Rev 21, 499–517 (2016). https://doi.org/10.1007/s10741-016-9561-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-016-9561-8