Abstract
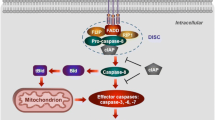
As cardiomyocytes have a limited capability for proliferation, renewal, and repair, the loss of heart cells followed by replacement with fibrous tissue is considered to result in the development of ventricular dysfunction and progression to heart failure (HF). The loss of cardiac myocytes in HF has been traditionally believed to occur mainly due to programmed apoptosis or unregulated necrosis. While extensive research work is being carried out to define the exact significance and contribution of both these cell death modalities in the development of HF, recent knowledge has indicated the existence and importance of a different form of cell death called necroptosis in the failing heart. This new cell damaging process, resembling some of the morphological features of passive necrosis as well as maladaptive autophagy, is a programmed process and is orchestrated by a complex set of proteins involving receptor-interacting protein kinase 1 and 3 (RIP1, RIP3) and mixed lineage kinase domain-like protein (MLKL). Activation of the RIP1–RIP3–MLKL signaling pathway leads to disruption of cation homeostasis, plasma membrane rupture, and finally cell death. It seems likely that inhibition of any site in this pathway may prove as an effective pharmacological intervention for preventing the necroptotic cell death in the failing heart. This review is intended to describe general aspects of the signaling pathway associated with necroptosis, to describe its relationship with cardiac dysfunction in some models of cardiac injury and discuss its potential relevance in various types of HF with respect to the underlying pathologic mechanisms.


Similar content being viewed by others
References
Olson RE (1959) Myocardial metabolism in congestive heart failure. J Chronic Dis 9:442–464
Dhalla NS, Das PK, Sharma GP (1978) Subcellular basis of cardiac contractile failure. J Mol Cell Cardiol 10:363–385
Packer M (1988) Neurohormonal interactions and adaptations in congestive heart failure. Circulation 77:721–730
Weber KT, Brilla CG (1991) Pathological hypertrophy and the cardiac interstitium: fibrosis and the renin-angiotensin-aldosterone system. Circulation 83:1849–1865
Gaudron P, Eilles C, Kugler I, Ertl G (1993) Progressive left ventricular dysfunction and remodeling after myocardial infarction. Circulation 87:755–763
Struijker-Boudier HAJ, Smits JFM, DeMey JGR (1995) Pharmacology of cardiac and vascular remodeling. Ann Rev Pharmacol Toxicol 35:509–539
Cohn JN, Ferrari R, Sharpe N (2000) Cardiac remodeling—concepts and clinical implications: a consensus paper from an international forum on cardiovascular remodeling. J Am Coll Cardiol 35:569–582
Mudd JO, Kass DA (2008) Tackling heart failure in the twenty-first century. Nature 451:919–928
Dhalla NS, Saini-Chohan HK, Rodriguez-Leyva D, Elimban V, Dent MR, Tappia PS (2009) Subcellular remodeling may induce cardiac dysfunction in congestive heart failure. Cardiovasc Res 81:429–438
Müller AL, Dhalla NS (2012) Role of various proteases in cardiac remodeling and progression of heart failure. Heart Fail Rev 17:395–409
Dhalla NS, Rangi S, Babick AP, Zieroth S, Elimban V (2012) Cardiac remodeling and subcellular defects in heart failure due to myocardial infarction and aging. Heart Fail Rev 17:671–681
Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, Murabito JM, Vasan RS (2002) Long-term trends in the incidence of and survival with heart failure. N Engl J Med 347:1397–1402
Goldberg RJ, Ciampa J, Lessard D, Meyer TE, Spencer FA (2007) Long-term survival after heart failure: a contemporary population-based perspective. Arch Intern Med 167:490–496
Bleumink GS, Knetsch AM, Sturkenboom MC, Straus SM, Hofman A, Deckers JW, Witteman JC, Stricker BH (2004) Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure. The Rotterdam Study. Eur Heart J 25:1614–1619
Dhalla NS, Temsah RM (2001) Sarcoplasmic reticulum and cardiac oxidative stress: an emerging target for heart disease. Expert Opin Ther Targets 5:205–217
Dhalla NS, Afzal N, Beamish RE, Naimark B, Takeda N, Nagano M (1993) Pathophysiology of cardiac dysfunction in congestive heart failure. Can J Cardiol 9:873–887
Dhalla NS, Temsah RM, Netticadan T (2000) Role of oxidative stress in cardiovascular diseases. J Hypertens 18(6):655–673
Hafstad AD, Nabeebaccus AA, Shah AM (2013) Novel aspects of ROS signalling in heart failure. Basic Res Cardiol 108:359
Dhalla NS, Dent MR, Tappia PS, Sethi R, Barta J, Goyal RK (2006) Subcellular remodeling as a viable target for the treatment of congestive heart failure. J Cardiovasc Pharmacol Ther 11:31–45
Olivetti G, Giordano G, Corradi D, Melissari M, Lagrasta C, Gambert SR, Anversa P (1995) Gender differences and aging: effects on the human heart. J Am Coll Cardiol 26:1068–1079
Smith DK, Zhang CL (2015) Regeneration through reprogramming adult cell identity in vivo. Am J Pathol 185:2619–2628
Kajstura J, Leri A, Finato N, Di Loreto C, Beltrami CA, Anversa P (1998) Myocyte proliferation in end-stage cardiac failure in humans. Proc Natl Acad Sci USA 95:8801–8805
Edinger AL, Thompson CB (2004) Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol 16:663–669
Nikoletopoulou V, Markaki M, Palikaras K, Tavernarakis N (2013) Crosstalk between apoptosis, necrosis and autophagy. Biochim Biophys Acta 1833:3448–3459
Klionsky DJ, Emr SD (2000) Autophagy as a regulated pathway of cellular degradation. Science 290:1717–1721
Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J (2005) Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 1:112–119
Miao B, Degterev A (2009) Methods to analyze cellular necroptosis. Methods Mol Biol 559:79–93
Luedde M, Lutz M, Carter N, Sosna J, Jacoby C, Vucur M, Gautheron J, Roderburg C, Borg N, Reisinger F, Hippe HJ, Linkermann A, Wolf MJ, Rose-John S, Lüllmann-Rauch R, Adam D, Flögel U, Heikenwalder M, Luedde T, Frey N (2014) RIP3, a kinase promoting necroptotic cell death, mediates adverse remodelling after myocardial infarction. Cardiovasc Res 103:206–216
Oerlemans MI, Liu J, Arslan F, den Ouden K, van Middelaar BJ, Doevendans PA, Sluijter JP (2012) Inhibition of RIP1-dependent necrosis prevents adverse cardiac remodeling after myocardial ischemia-reperfusion in vivo. Basic Res Cardiol 107:270
Smith CC, Davidson SM, Lim SY, Simpkin JC, Hothersall JS, Yellon DM (2007) Necrostatin: a potentially novel cardioprotective agent? Cardiovasc Drugs Ther 21:227–233
Berghe TV, Vanlangenakker N, Parthoens E, Deckers W, Devos M, Festjens N, Guerin CJ, Brunk UT, Declercq W, Vandenabeele P (2010) Necroptosis, necrosis and secondary necrosis converge on similar cellular disintegration features. Cell Death Differ 17:922–930
Berghe TV, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P (2014) Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol 15:135–147
Boya P, Reggiori F, Codogno P (2013) Emerging regulation and functions of autophagy. Nat Cell Biol 15(7):713–720
Takemura G, Miyata S, Kawase Y, Okada H, Maruyama R, Fujiwara H (2006) Autophagic degeneration and death of cardiomyocytes in heart failure. Autophagy 2:212–214
Hariharan N, Zhai P, Sadoshima J (2011) Oxidative stress stimulates autophagic flux during ischemia/reperfusion. Antioxid Redox Signal 14:2179–2190
Ma X, Liu H, Foyil SR, Godar RJ, Weinheimer CJ, Hill JA, Diwan A (2012) Impaired autophagosome clearance contributes to cardiomyocyte death in ischemia/reperfusion injury. Circulation 125:3170–3181
Sciarretta S, Hariharan N, Monden Y, Zablocki D, Sadoshima J (2010) Is autophagy in response to ischemia and reperfusion protective or detrimental for the heart? Pediatr Cardiol 32:275–281
Basit F, Cristofanon S, Fulda S (2013) Obatoclax (GX15-070) triggers necroptosis by promoting the assembly of the necrosome on autophagosomal membranes. Cell Death Differ 20:1161–1173
Guerra S, Leri A, Wang X, Finato N, Di Loreto C, Beltrami CA, Kajstura J, Anversa P (1999) Myocyte death in the failing human heart is gender dependent. Circ Res 85:856–866
Kang PM, Izumo S (2000) Apoptosis and heart failure: a critical review of the literature. Circ Res 86:1107–1113
Mani K (2008) Programmed cell death in cardiac myocytes: strategies to maximize post-ischemic salvage. Heart Fail Rev 13:193–209
Knaapen MW, Davies MJ, De Bie M, Haven AJ, Martinet W, Kockx MM (2001) Apoptotic versus autophagic cell death in heart failure. Cardiovasc Res 51:304–312
Nagarajan V, Hernandez AV, Tang WH (2012) Prognostic value of cardiac troponin in chronic stable heart failure: a systematic review. Heart 98:1778–1786
Kung G, Konstantinidis K, Kitsis RN (2011) Programmed necrosis, not apoptosis in the heart. Circ Res 108:1017–1036
Krysko DV, Berghe TV, D'Herde K, Vandenabeele P (2008) Apoptosis and necrosis: detection, discrimination and phagocytosis. Methods 44:205–221
Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M, Cain K, MacFarlane M, Hacker G, Leverkus M (2011) cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Mol Cell 43:449–463
Dillon CP, Oberst A, Weinlich R, Janke LJ, Kang TB, Ben-Moshe T, Mak TW, Wallach D, Green DR (2012) Survival function of the FADD-CASPASE-8-cFLIP(L) complex. Cell Rep 1:401–407
Osborn SL, Diehl G, Han SJ, Xue L, Kurd N, Hsieh K, Cado D, Robey EA, Winoto A (2010) Fas-associated death domain (FADD) is a negative regulator of T-cell receptor-mediated necroptosis. Proc Natl Acad Sci USA 107:13034–13039
Wang H, Sun L, Su L, Rizo J, Liu L, Wang LF, Wang FS, Wang X (2014) Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell 54:133–146
Colbert LE, Fisher SB, Hardy CW, Hall WA, Saka B, Shelton JW, Petrova AV, Warren MD, Pantazides BG, Gandhi K, Kowalski J, Kooby DA, El-Rayes BF, Staley CA 3rd, Adsay NV, Curran WJ Jr, Landry JC, Maithel SK, Yu DS (2013) Pronecrotic mixed lineage kinase domain-like protein expression is a prognostic biomarker in patients with early-stage resected pancreatic adenocarcinoma. Cancer 119:3148–3155
Pierdomenico M, Negroni A, Stronati L, Vitali R, Prete E, Bertin J, Gough PJ, Aloi M, Cucchiara S (2014) Necroptosis is active in children with inflammatory bowel disease and contributes to heighten intestinal inflammation. Am J Gastroenterol 109:279–287
Mizumura K, Cloonan SM, Nakahira K, Bhashyam AR, Cervo M, Kitada T, Glass K, Owen CA, Mahmood A, Washko GR, Hashimoto S, Ryter SW, Choi AM (2014) Mitophagy-dependent necroptosis contributes to the pathogenesis of COPD. J Clin Invest 124:3987–4003
Desai J, Vr SK, Mulay SR, Konrad L, Romoli S, Schauer C, Herrmann M, Bilyy R, Müller S, Popper B, Nakazawa D, Weidenbusch M, Thomasova D, Krautwald S, Linkermann A, Anders HJ (2015) Neutrophil extracellular trap formation can involve RIPK1-RIPK3-MLKL signalling. Eur J Immunol. doi:10.1002/eji.201545605
Kitur K, Parker D, Nieto P, Ahn DS, Cohen TS, Chung S, Wachtel S, Bueno S, Prince A (2015) Toxin-induced necroptosis is a major mechanism of Staphylococcus aureus lung damage. PLoS Pathog 11:e1004820
Omoto S, Guo H, Talekar GR, Roback L, Kaiser WJ, Mocarski ES (2015) Suppression of RIP3-dependent necroptosis by human cytomegalovirus. J Biol Chem 290:11635–11648
Moquin D, Chan FK (2010) The molecular regulation of programmed necrotic cell injury. Trends Biochem Sci 35:434–441
Davis CW, Hawkins BJ, Ramasamy S, Irrinki KM, Cameron BA, Islam K, Daswani VP, Doonan PJ, Manevich Y, Madesh M (2010) Nitration of the mitochondrial complex I subunit NDUFB8 elicits RIP1- and RIP3-mediated necrosis. Free Radic Biol Med 15(48):306–317
Kim S, Dayani L, Rosenberg PA, Li J (2010) RIP1 kinase mediates arachidonic acid-induced oxidative death of oligodendrocyte precursors. Int J Physiol Pathophysiol Pharmacol 2:137–147
Han W, Li L, Qiu S, Lu Q, Pan Q, Gu Y, Luo J, Hu X (2007) Shikonin circumvents cancer drug resistance by induction of a necroptotic death. Mol Cancer Ther 6:1641–1649
Micheau O, Tschopp J (2003) Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114:181–190
Schneider-Brachert W, Tchikov V, Neumeyer J, Jakob M, Winoto-Morbach S, Held-Feindt J, Heinrich M, Merkel O, Ehrenschwender M, Adam D, Mentlein R, Kabelitz D, Schütze S (2004) Compartmentalization of TNF receptor 1 signaling: internalized TNF receptosomes as death signaling vesicles. Immunity 21:415–428
Li H, Kobayashi M, Blonska M, You Y, Lin X (2006) Ubiquitination of RIP is required for tumor necrosis factor alpha-induced NF-kappaB activation. J Biol Chem 281:13636–13643
Ea CK, Deng L, Xia ZP, Pineda G, Chen ZJ (2006) Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell 22:245–257
O'Donnell MA, Legarda-Addison D, Skountzos P, Yeh WC, Ting AT (2007) Ubiquitination of RIP1 regulates an NF-kappaB-independent cell-death switch in TNF signaling. Curr Biol 17:418–424
Moquin DM, McQuade T, Chan FK (2013) CYLD deubiquitinates RIP1 in the TNFα-induced necrosome to facilitate kinase activation and programmed necrosis. PLoS One 8:e76841
Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR (2011) Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature 471:363–367
Kataoka T (2005) The caspase-8 modulator c-FLIP. Crit Rev Immunol 25:31–58
Scaffidi C, Schmitz I, Krammer PH, Peter ME (1999) The role of c-FLIP in modulation of CD95-induced apoptosis. J Biol Chem 274:1541–1548
Stennicke HR, Jürgensmeier JM, Shin H, Deveraux Q, Wolf BB, Yang X, Zhou Q, Ellerby HM, Ellerby LM, Bredesen D, Green DR, Reed JC, Froelich CJ, Salvesen GS (1998) Pro-caspase-3 is a major physiologic target of caspase-8. J Biol Chem 273:27084–27090
Kavuri SM, Geserick P, Berg D, Dimitrova DP, Feoktistova M, Siegmund D, Gollnick H, Neumann M, Wajant H, Leverkus M (2011) Cellular FLICE- inhibitory protein (cFLIP) isoforms block CD95- and TRAIL death receptor-induced gene induction irrespective of processing of caspase-8 or cFLIP in the death-inducing signaling complex. J Biol Chem 286:16631–16646
Cho YS, Challa S, Moquin D et al (2009) Phosphorylation-driven assembly of the RIP1–RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137:1112–1123
Li J, McQuade T, Siemer AB, Napetschnig J, Moriwaki K, Hsiao YS, Damko E, Moquin D, Walz T, McDermott A, Chan FK, Wu H (2012) The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell 150:339–350
Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X (2012) Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148:213–227
Wu J, Huang Z, Ren J, Zhang Z, He P, Li Y, Ma J, Chen W, Zhang Y, Zhou X, Yang Z, Wu SQ, Chen L, Han J (2013) Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res 23:994–1006
Zhao J, Jitkaew S, Cai Z, Choksi S, Li Q, Luo J, Liu ZG (2012) Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci USA 109:5322–5327
Cai Z, Jitkaew S, Zhao J, Chiang HC, Choksi S, Liu J, Ward Y, Wu LG, Liu ZG (2014) Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol 16:55–65
Chen X, Li W, Ren J, Huang D, He WT, Song Y, Yang C, Li W, Zheng X, Chen P, Han J (2014) Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res 24:105–121
Dondelinger Y, Declercq W, Montessuit S, Roelandt R, Goncalves A, Bruggeman I, Hulpiau P, Weber K, Sehon CA, Marquis RW, Bertin J, Gough PJ, Savvides S, Martinou JC, Betrand MJ, Vandenabeele P (2014) MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep 7:971–981
Degterev A, Hitomi J, Germscheid M, Ch'en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, Hedrick SM, Gerber SA, Lugovskoy A, Yuan J (2008) Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol 4:313–321
Takahashi N, Duprez L, Grootjans S, Cauwels A, Nerinckx W, DuHadaway JB, Goossens V, Roelandt R, Van Hauwermeiren F, Libert C, Declercq W, Callewaert N, Prendergast GC, Degterev A, Yuan J, Vandenabeele P (2012) Necrostatin-1 analogues: critical issues on the specificity, activity and in vivo use in experimental disease models. Cell Death Dis 3:e437
Hescheler J, Meyer R, Plant S, Krautwurst D, Rosenthal W, Schultz G (1991) Morphological, biochemical, and electrophysiological characterization of a clonal cell (H9c2) line from rat heart. Circ Res 69:1476–1486
Szobi A, Rajtik T, Carnicka S, Ravingerova T, Adameova A (2014) Mitigation of postischemic cardiac contractile dysfunction by CaMKII inhibition: effects on programmed necrotic and apoptotic cell death. Mol Cell Biochem 388:269–276
Dmitriev YV, Minasian SM, Demchenko EA, Galagudza MM (2013) Study of cardioprotective effects of necroptosis inhibitors on isolated rat heart subjected to global ischemia-reperfusion. Bull Exp Biol Med 155:245–248
Koshinuma S, Miyamae M, Kaneda K, Kotani J, Figueredo VM (2014) Combination of necroptosis and apoptosis inhibition enhances cardioprotection against myocardial ischemia-reperfusion injury. J Anesth 28:235–241
Koudstaal S, Oerlemans MI, Van der Spoel TI, Janssen AW, Hoefer IE, Doevendans PA, Sluijter JP, Chamuleau SA (2015) Necrostatin-1 alleviates reperfusion injury following acute myocardial infarction in pigs. Eur J Clin Invest 45:150–159
Lim SY, Davidson SM, Mocanu MM, Yellon DM, Smith CC (2007) The cardioprotective effect of necrostatin requires the cyclophilin-D component of the mitochondrial permeability transition pore. Cardiovasc Drugs Ther 21:467–469
Meng L, Jin W, Wang X (2015) RIP3-mediated necrotic cell death accelerates systematic inflammation and mortality. Proc Natl Acad Sci USA 112:11007–11012
Kleinbongard P, Schulz R, Heusch G (2011) TNFα in myocardial ischemia/reperfusion, remodeling and heart failure. Heart Fail Rev 16:49–69
Sun M, Dawood F, Wen WH, Chen M, Dixon I, Kirshenbaum LA, Liu PP (2004) Excessive tumor necrosis factor activation after infarction contributes to susceptibility of myocardial rupture and left ventricular dysfunction. Circulation 110:3221–3228
Lin Y, Choksi S, Shen HM, Yang QF, Hur GM, Kim YS, Tran JH, Nedospasov SA, Liu ZG (2004) Tumor necrosis factor-induced nonapoptotic cell death requires receptor-interacting protein-mediated cellular reactive oxygen species accumulation. J Biol Chem 279:10822–10828
He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X (2009) Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell 137:1100–1111
Chen Y, Pat B, Zheng J, Cain L, Powell P, Shi K, Sabri A, Husain A, Dell'italia LJ (2010) Tumor necrosis factor-α produced in cardiomyocytes mediates a predominant myocardial inflammatory response to stretch in early volume overload. J Mol Cell Cardiol 49:70–78
Chen Y, Pat B, Gladden JD, Zheng J, Powell P, Wei CC, Cui X, Husain A, Dell'italia LJ (2011) Dynamic molecular and histopathological changes in the extracellular matrix and inflammation in the transition to heart failure in isolated volume overload. Am J Physiol Heart Circ Physiol 300:H2251–H2260
Hayashi Y, Hanawa H, Jiao S, Hasegawa G, Ohno Y, Yoshida K, Suzuki T, Kashimura T, Obata H, Tanaka K, Watanabe T, Minamino T (2015) Elevated endomyocardial biopsy macrophage-related markers in intractable myocardial diseases. Inflammation 38:2288–2299
Sakaguchi H, Yamamoto T, Ono S, Akashi A, Tsuda E, Watanabe K (2012) An infant case of dilated cardiomyopathy associated with congenital cytomegalovirus infection. Pediatr Cardiol 33:824–826
Partanen J, Nieminen MS, Krogerus L, Lautenschlager I, Geagea A, Aarnio P, Mattila S (1991) Cytomegalovirus myocarditis in transplanted heart verified by endomyocardial biopsy. Clin Cardiol 14:847–849
Upton JW, Kaiser WJ, Mocarski ES (2010) Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe 7:302–313
Zhang DW, Zheng M, Zhao J, Li YY, Huang Z, Li Z, Han J (2011) Multiple death pathways in TNF-treated fibroblasts: RIP3- and RIP1-dependent and independent routes. Cell Res 21:368–371
Haunstetter A, Izumo S (2000) Toward antiapoptosis as a new treatment modality. Circ Res 86:371–376
Acknowledgments
The research in this article was supported by a grant from the Slovak Scientific Grant Agency (VEGA) VEGA 1/0638/12, 1/0271/16. The infrastructural support for this study was provided by the St. Boniface Hospital Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Drs. Adriana Adameova, Eva Goncalvesova, Adrian Szobi, and Prof. Naranjan Dhalla have no conflicts of interest or financial ties to disclose.
Rights and permissions
About this article
Cite this article
Adameova, A., Goncalvesova, E., Szobi, A. et al. Necroptotic cell death in failing heart: relevance and proposed mechanisms. Heart Fail Rev 21, 213–221 (2016). https://doi.org/10.1007/s10741-016-9537-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-016-9537-8