Abstract
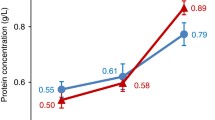
Hepatitis B is a potentially life-threatening liver infection caused by the hepatitis B virus (HBV), which can lead to chronic liver disease and put people at high risk of death from cirrhosis of the liver and liver cancer. However, little is known about the correlation of salivary N-linked glycans related to HBV-infected liver diseases. Here we investigated N-linked glycome in saliva from 200 subjects (50 healthy volunteers (HV), 40 HBV-infected patients (HB), 50 cirrhosis patients (HC), and 60 hepatocellular carcinoma patients (HCC) using MALDI-TOF/TOF-MS. Representative MS spectra of N-glycans with signal-to-noise ratios >6 were annotated using the GlycoWorkbench program. A total of 40, 47, 29, and 33 N-glycan peaks were identified and annotated from HV, HB, HC, and HCC groups, respectively. There were 15 N-glycan peaks (e.g., m/z 1647.587, 1688.613 and 2101.755) were present in all groups. Three N-glycan peaks (m/z 2596.925, 2756.962, and 2921.031) were unique in HV group, 2 N-glycan peaks (m/z 1898.676 and 1971.692) were unique in HB group, 5 N-glycan peaks (m/z 1954.677, 2507.914, 2580.930, 2637.952, and 3092.120) were unique in HC group, and 3 N-glycan peaks (m/z 2240.830, 2507.914, and 3931.338) were unique in HCC group. The proportion of fucosylated N-glycans was apparently increased in the HCC group (84.8%) than in any other group (73.1% ± 0.01), however, the proportion of sialylated N-glycans was decreased in HCC group (12.1%) than in any other group (17.23% ± 0.003). Our data provide pivotal information to distinguish between HBV-associated hepatitis, cirrhosis and HCC, and facilitate the discovery of biomarkers for HCC during its early stages based on precise alterations of N-linked glycans in saliva.




Similar content being viewed by others
References
Who.int: Hepatitis B. http://www.who.int/mediacentre/factsheets/fs204/en/ (2016)
Liu, J., Fan, D.: Hepatitis B in China. Lancet (London, England). 369(9573), 1582–1583 (2007)
Pfaffe, T., Cooper-White, J., Beyerlein, P., Kostner, K., Punyadeera, C.: Diagnostic potential of saliva: current state and future applications. Clin. Chem. 57(5), 675–687 (2011)
Javaid, M.A., Ahmed, A.S., Durand, R., Tran, S.D.: Saliva as a diagnostic tool for oral and systemic diseases. J. Oral. Biol. Craniofac. Res. 6(1), 66–75 (2016)
Foley 3rd, J.D., Sneed, J.D., Steinhubl, S.R., Kolasa, J., Ebersole, J.L., Lin, Y., Kryscio, R.J., McDevitt, J.T., Campbell, C.L., Miller, C.S.: Oral fluids that detect cardiovascular disease biomarkers. Oral Surg Oral Med Oral Pathol Oral Radiol. 114(2), 207–214 (2012)
Bencharit, S., Baxter, S.S., Carlson, J., Byrd, W.C., Mayo, M.V., Border, M.B., Kohltfarber, H., Urrutia, E., Howard-Williams, E.L., Offenbacher, S., Wu, M.C., Buse, J.B.: Salivary proteins associated with hyperglycemia in diabetes: a proteomic analysis. Mol. BioSyst. 9(11), 2785–2797 (2013)
Hu, S., Arellano, M., Boontheung, P., Wang, J., Zhou, H., Jiang, J., Elashoff, D., Wei, R., Loo, J.A., Wong, D.T.: Salivary proteomics for oral cancer biomarker discovery. Clin Cancer Res. 14(19), 6246–6252 (2008)
Zhang, L., Xiao, H., Karlan, S., Zhou, H., Gross, J., Elashoff, D., Akin, D., Yan, X., Chia, D., Karlan, B.: Discovery and preclinical validation of salivary transcriptomic and proteomic biomarkers for the non-invasive detection of breast cancer. PLoS One. 5(12), e15573–e15573 (2010)
Zhang, L., Xiao, H., Wong, D.T.: Salivary biomarkers for clinical applications. Mol Diagn Ther. 13(4), 245–259 (2009)
Li, D., Mallory, T., Satomura, S.: AFP-L3: a new generation of tumor marker for hepatocellular carcinoma. Clin Chim Acta. 313(1–2), 15–19 (2001)
Lebrilla, C.B., An, H.J.: The prospects of glycan biomarkers for the diagnosis of diseases. Mol. BioSyst. 5(1), 17–20 (2009)
Kratz, E.M., Waszkiewicz, N., Kaluza, A., Szajda, S.D., Zalewska-Szajda, B., Szulc, A., Zwierz, K., Ferens-Sieczkowska, M.: Glycosylation changes in the salivary glycoproteins of alcohol-dependent patients: a pilot study. Alcohol Alcohol. 49(1), 23–30 (2014)
Kozak, R.P., Urbanowicz, P.A., Punyadeera, C., Reiding, K.R., Jansen, B.C., Royle, L., Spencer, D.I., Fernandes, D.L., Wuhrer, M.: Variation of human salivary O-Glycome. PLoS One. 11(9), e0162824 (2016)
Qin, Y., Zhong, Y., Zhu, M., Dang, L., Yu, H., Chen, Z., Chen, W., Wang, X., Zhang, H., Li, Z.: Age- and sex-associated differences in the glycopatterns of human salivary glycoproteins and their roles against influenza a virus. J. Proteome Res. 12(6), 2742–2754 (2013)
Zhong, Y., Qin, Y., Yu, H., Yu, J., Wu, H., Lin, C., Zhang, P., Wang, X., Jia, Z., Guo, Y.: Avian influenza virus infection risk in humans with chronic diseases. Sci Rep. 5, 8971 (2014)
Yang, G.L., Tian-Ran, M.A., Zheng, L.I.: Enrichment and characterization of Total N-linked Glycans from glycoproteins by ultrafiltration units and mass spectrometry. Prog Biochem Biophys. 41(4), 403–408 (2014)
Yang, G., Tan, Z., Lu, W., Guo, J., Yu, H., Yu, J., Sun, C., Qi, X., Li, Z., Guan, F.: Quantitative glycome analysis of N-glycan patterns in bladder cancer vs normal bladder cells using an integrated strategy. J. Proteome Res. 14(2), 639–653 (2015)
Yang, G., Cui, T., Wang, Y., Sun, S., Ma, T., Wang, T., Chen, Q., Li, Z.: Selective isolation and analysis of glycoprotein fractions and their glycomes from hepatocellular carcinoma sera. Proteomics. 13(9), 1481–1498 (2013)
Tan, Z., Lu, W., Li, X., Yang, G., Guo, J., Yu, H., Li, Z., Guan, F.: Altered N-glycan expression profile in epithelial-to-mesenchymal transition of NMuMG cells revealed by an integrated strategy using mass spectrometry and glycogene and lectin microarray analysis. J. Proteome Res. 13(6), 2783–2795 (2014)
Reis, C.A., Osorio, H., Silva, L., Gomes, C., David, L.: Alterations in glycosylation as biomarkers for cancer detection. J. Clin. Pathol. 63(4), 322–329 (2010)
Kam, R.K.T., Poon, T.C.W.: The potentials of Glycomics in biomarker discovery. Clin. Proteomics. 4(3), 67–79 (2008)
Yu, H., Zhu, M., Qin, Y., Zhong, Y., Yan, H., Wang, Q., Bian, H., Li, Z.: Analysis of glycan-related genes expression and glycan profiles in mice with liver fibrosis. J. Proteome Res. 11(11), 5277–5285 (2012)
Vajaria, B.N., Patel, P.S.: Glycosylation: a hallmark of cancer? Glycoconj J. 34(2), 147–156 (2017)
Qin, Y., Zhong, Y., Ma, T., Wu, F., Wu, H., Yu, H., Huang, C., Li, Z.: Alteration of liver glycopatterns during cirrhosis and tumor progression induced by HBV. Glycoconj. J. 33(2), 125–136 (2016)
Blomme, B., Van Steenkiste, C., Callewaert, N., Van Vlierberghe, H.: Alteration of protein glycosylation in liver diseases. J. Hepatol. 50(3), 592–603 (2009)
Aoyagi, Y., Suzuki, Y., Igarashi, K., Saitoh, A., Oguro, M., Yokota, T., Mori, S., Suda, T., Isemura, M., Asakura, H.: Carbohydrate structures of human alpha-fetoprotein of patients with hepatocellular carcinoma: presence of fucosylated and non-fucosylated triantennary glycans. Br. J. Cancer. 67(3), 486–492 (1993)
Aoyagi, Y., Isokawa, O., Suda, T., Watanabe, M., Suzuki, Y., Asakura, H.: The fucosylation index of alpha-fetoprotein as a possible prognostic indicator for patients with hepatocellular carcinoma. Cancer. 83(10), 2076–2082 (1998)
Vanhooren, V., Liu, X.E., Franceschi, C., Gao, C.F., Libert, C., Contreras, R., Chen, C.: N-glycan profiles as tools in diagnosis of hepatocellular carcinoma and prediction of healthy human ageing. Mech. Ageing Dev. 130(1–2), 92–97 (2009)
Pompach, P., Ashline, D.J., Brnakova, Z., Benicky, J., Sanda, M., Goldman, R.: Protein and site specificity of fucosylation in liver-secreted glycoproteins. J. Proteome Res. 13(12), 5561–5569 (2014)
Kudo, T., Nakagawa, H., Takahashi, M., Hamaguchi, J., Kamiyama, N., Yokoo, H., Nakanishi, K., Nakagawa, T., Kamiyama, T., Deguchi, K., Nishimura, S., Todo, S.: N-glycan alterations are associated with drug resistance in human hepatocellular carcinoma. Mol. Cancer. 6, 32 (2007)
Kamiyama, T., Yokoo, H., Furukawa, J., Kurogochi, M., Togashi, T., Miura, N., Nakanishi, K., Kamachi, H., Kakisaka, T., Tsuruga, Y., Fujiyoshi, M., Taketomi, A., Nishimura, S., Todo, S.: Identification of novel serum biomarkers of hepatocellular carcinoma using glycomic analysis. Hepatology (Baltimore, Md.) 57(6), 2314–2325 (2013)
Goldman, R., Ressom, H.W., Varghese, R.S., Goldman, L., Bascug, G., Loffredo, C.A., Abdelhamid, M., Gouda, I., Ezzat, S., Kyselova, Z.: Detection of hepatocellular carcinoma using Glycomic analysis. Clin Cancer Res. 15(5), 1808–1813 (2009)
Noda, K., Miyoshi, E., Kitada, T., Nakahara, S., Gao, C.X., Honke, K., Shiratori, Y., Moriwaki, H., Sasaki, Y., Kasahara, A., Hori, M., Hayashi, N., Taniguchi, N.: The enzymatic basis for the conversion of nonfucosylated to fucosylated alpha-fetoprotein by acyclic retinoid treatment in human hepatoma cells: activation of alpha1-6 fucosyltransferase. Tumour Biol. 23(4), 202–211 (2002)
Mehta, A., Block, T.M.: Fucosylated glycoproteins as markers of liver disease. Dis. Markers. 25(4–5), 259–265 (2008)
Comunale, M.A., Rodemichbetesh, L., Hafner, J., Wang, M., Norton, P., Bisceglie, A.M.D., Block, T., Mehta, A.: Linkage specific Fucosylation of alpha-1-antitrypsin in liver Cirrhosis and cancer patients: implications for a biomarker of hepatocellular carcinoma. PLoS One. 5(8), 521–526 (2010)
Comunale, M.A., Lowman, M., Long, R.E., Krakover, J., Philip, R., Seeholzer, S., Evans, A.A., Hann, H.W., Block, T.M., Mehta, A.S.: Proteomic analysis of serum associated fucosylated glycoproteins in the development of primary hepatocellular carcinoma. J. Proteome Res. 5(2), 308–315 (2006)
Aoyagi, Y., Isemura, M., Yosizawa, Z., Suzuki, Y., Sekine, C., Ono, T., Ichida, F.: Fucosylation of serum alpha-fetoprotein in patients with primary hepatocellular carcinoma. Biochim. Biophys. Acta. 830(3), 217–223 (1985)
Vajaria, B.N., Patel, K.R., Begum, R., Patel, P.S.: Sialylation: an avenue to target cancer cells. Pathol Oncol Res : POR. 22(3), 443–447 (2016)
Mehta, A., Herrera, H., Block, T.: Glycosylation and liver cancer. Adv. Cancer Res. 126, 257–279 (2015)
Dall'Olio, F., Chiricolo, M., D'Errico, A., Gruppioni, E., Altimari, A., Fiorentino, M., Grigioni, W.F.: Expression of β-galactoside α2,6 sialyltransferase and of α2,6-sialylated glycoconjugates in normal human liver, hepatocarcinoma, and cirrhosis. Glycobiology. 14(1), 39–49 (2004)
Yamashita, K., Tachibana, Y., Nakayama, T., Kitamura, M., Endo, Y., Kobata, A.: Structural studies of the sugar chains of human parotid alpha-amylase. J. Biol. Chem. 255(12), 5635–5642 (1980)
Takashima, S., Amano, J.: Glycosylation and secretion of human α-amylases. Advances in Biological Chemistry. 2(1), 10–19 (2012)
Acknowledgements
This work is supported by the National Natural Science Foundation (Grant No. 81372365).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article is conducted in accordance with the Ethical Guidelines of the Declaration of Helsinki.
Rights and permissions
About this article
Cite this article
Qin, Y., Zhong, Y., Ma, T. et al. A pilot study of salivary N-glycome in HBV-induced chronic hepatitis, cirrhosis, and hepatocellular carcinoma. Glycoconj J 34, 523–535 (2017). https://doi.org/10.1007/s10719-017-9768-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-017-9768-5