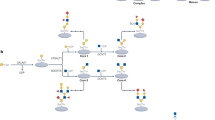
Abstract
Cardiovascular diseases arising from atherosclerosis are currently the leading cause of mortality worldwide. Leukocyte recruitment is a key step for the successful initiation of atherosclerosis and occurs predominantly in the inflamed endothelium. Leukocyte recruitment is mediated by a group of adhesive molecules and chemokine receptors, which are often glycosylated protein. Recent studies demonstrated that post-translational glycosylation by glycosyltransferases is necessary for adhesive molecules and chemokine receptors activities. Several glycosyltransferases, such as α2,3-sialyltransferases IV, α1,3-fucosyltransferases IV and VII, core 2 β1,6-N-acetylglucosaminyltransferase-I, are considered to participate in the synthesis of glycosylation for adhesive molecules and chemokine receptors, and the initiation of atherosclerotic lesions. In this review, we will discuss new data concerning the roles of different glycosyltransferases in atherogenesis. The knowledge of glycosyltransferases in atherogenesis offers the opportunity to develop novel therapeutic strategies.


Similar content being viewed by others
References
Hansson, G.K.: Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 352(16), 1685–1695 (2005)
Libby, P., Ridker, P.M., Maseri, A.: Inflammation and atherosclerosis. Circulation 105(9), 1135–1143 (2002)
Ross, R.: The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362(6423), 801–809 (1993)
Huo, Y., Ley, K.: Adhesion molecules and atherogenesis. Acta Physiol. Scand. 173(1), 35–43 (2001)
Blankenberg, S., Barbaux, S., Tiret, L.: Adhesion molecules and atherosclerosis. Atherosclerosis 170(2), 191–203 (2003)
Tschoepe, D.: Adhesion molecules influencing atherosclerosis. Diabetes Res. Clin. Pract. 30, S19–S24 (1996)
Lowe, J.B.: Glycosyltransferases and glycan structures contributing to the adhesive activities of L-, E- and P-selectin counter-receptors. Biochem. Soc. Symp. (69), 33–45 (2002).
Jongstra-Bilen, J., Haidari, M., Zhu, S.N., Chen, M., Guha, D., Cybulsky, M.I.: Low-grade chronic inflammation in regions of the normal mouse arterial intima predisposed to atherosclerosis. J. Exp. Med. 203(9), 2073–2083 (2006)
Skalen, K., Gustafsson, M., Rydberg, E.K., Hulten, L.M., Wiklund, O., Innerarity, T.L., Boren, J.: Subendothelial retention of atherogenic lipoproteins in early atherosclerosis. Nature 417(6890), 750–754 (2002)
Oorni, K., Posio, P., Ala-Korpela, M., Jauhiainen, M., Kovanen, P.T.: Sphingomyelinase induces aggregation and fusion of small very low-density lipoprotein and intermediate-density lipoprotein particles and increases their retention to human arterial proteoglycans. Arterioscler. Thromb. Vasc. Biol. 25(8), 1678–1683 (2005)
Williams, K.J., Fau-Tabas, I., Tabas, I.: Lipoprotein retention–and clues for atheroma regression. Arterioscler. Thromb. Vasc. Biol. 25(8), 1536–1540 (2005)
Moore, K.J., Sheedy, F.J., Fisher, E.A.: Macrophages in atherosclerosis: a dynamic balance. Nat. Rev. Immunol. 13(10), 709–721 (2013)
Khan, B., Parthasarathy, S., Alexander, R., Medford, R.: Modified low density lipoprotein and its constituents augment cytokine-activated vascular cell adhesion molecule-1 gene expression in human vascular endothelial cells. J. Clin. Investig. 95(3), 1262 (1995)
Kita, T., Kume, N., Minami, M., Hayashida, K., Murayama, T., Sano, H., Moriwaki, H., Kataoka, H., Nishi, E., Horiuchi, H.: Role of oxidized LDL in atherosclerosis. Ann. N. Y. Acad. Sci. 947(1), 199–206 (2001)
Kaperonis, E.A., Liapis, C.D., Kakisis, J.D., Dimitroulis, D., Papavassiliou, V.G.: Inflammation and atherosclerosis. Eur. J. Vasc. Endovasc. Surg. 31(4), 386–393 (2006)
Tuttolomondo, A., Di Raimondo, D., Pecoraro, R., Arnao, V., Pinto, A., Licata, G.: Atherosclerosis as an inflammatory disease. Curr. Pharm. Des. 18(28), 4266–4288 (2012)
Braunersreuther, V., Mach, F., Steffens, S.: The specific role of chemokines in atherosclerosis. Thromb. Haemost. 97(5), 714–721 (2007)
Eriksson, E.E., Xie, X., Werr, J., Thoren, P., Lindbom, L.: Importance of primary capture and L-selectin-dependent secondary capture in leukocyte accumulation in inflammation and atherosclerosis in vivo. J. Exp. Med. 194(2), 205–218 (2001)
Kansas, G.S.: Selectins and their ligands: current concepts and controversies. Blood 88(9), 3259–3287 (1996)
Rosen, S.D., Bertozzi, C.R.: The selectins and their ligands. Curr. Opin. Cell Biol. 6(5), 663–673 (1994)
Vestweber, D.: The selectins and their ligands. Curr. Top. Microbiol. Immunol. 184, 65–75 (1993)
Ley, K., Bullard, D.C., Arbones, M.L., Bosse, R., Vestweber, D., Tedder, T.F., Beaudet, A.L.: Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J. Exp. Med. 181(2), 669–675 (1995)
Bevilacqua, M.P., Pober, J.S., Mendrick, D.L., Cotran, R.S., Gimbrone Jr., M.A.: Identification of an inducible endothelial-leukocyte adhesion molecule. Proc. Natl. Acad. Sci. U. S. A. 84(24), 9238–9242 (1987)
An, G., Wang, H., Tang, R., Yago, T., McDaniel, J.M., McGee, S., Huo, Y., Xia, L.: P-selectin glycoprotein ligand-1 is highly expressed on Ly-6Chi monocytes and a major determinant for Ly-6Chi monocyte recruitment to sites of atherosclerosis in mice. Circulation 117(25), 3227–3237 (2008)
Collins, R.G., Velji, R., Guevara, N.V., Hicks, M.J., Chan, L., Beaudet, A.L.: P-Selectin or intercellular adhesion molecule (ICAM)-1 deficiency substantially protects against atherosclerosis in apolipoprotein E-deficient mice. J. Exp. Med. 191(1), 189–194 (2000)
Dong, Z.M., Brown, A.A., Wagner, D.D.: Prominent role of P-selectin in the development of advanced atherosclerosis in ApoE-deficient mice. Circulation 101(19), 2290–2295 (2000)
Dong, Z.M., Chapman, S.M., Brown, A.A., Frenette, P.S., Hynes, R.O., Wagner, D.D.: The combined role of P- and E-selectins in atherosclerosis. J. Clin. Invest. 102(1), 145–152 (1998)
Phillips, J.W., Barringhaus, K.G., Sanders, J.M., Hesselbacher, S.E., Czarnik, A.C., Manka, D., Vestweber, D., Ley, K., Sarembock, I.J.: Single injection of P-selectin or P-selectin glycoprotein ligand-1 monoclonal antibody blocks neointima formation after arterial injury in apolipoprotein E-deficient mice. Circulation 107(17), 2244–2249 (2003)
Rozenberg, I., Sluka, S.H., Mocharla, P., Hallenberg, A., Rotzius, P., Boren, J., Krankel, N., Landmesser, U., Borsig, L., Luscher, T.F., Eriksson, E.E., Tanner, F.C.: Deletion of L-selectin increases atherosclerosis development in ApoE-/- mice. PLoS One 6(7), e21675 (2011)
Reape, T.J., Groot, P.H.: Chemokines and atherosclerosis. Atherosclerosis 147(2), 213–225 (1999)
Zernecke, A., Weber, C.: Chemokines in the vascular inflammatory response of atherosclerosis. Cardiovasc. Res. 86(2), 192–201 (2010)
Tacke, F., Alvarez, D., Kaplan, T.J., Jakubzick, C., Spanbroek, R., Llodra, J., Garin, A., Liu, J., Mack, M., van Rooijen, N.: Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Invest. 117(1), 185–194 (2007)
Heller, E.A., Liu, E., Tager, A.M., Yuan, Q., Lin, A.Y., Ahluwalia, N., Jones, K., Koehn, S.L., Lok, V.M., Aikawa, E.: Chemokine CXCL10 promotes atherogenesis by modulating the local balance of effector and regulatory T cells. Circulation 113(19), 2301–2312 (2006)
Van Wanrooij, E.J., de Jager, S.C., van Es, T., de Vos, P., Birch, H.L., Owen, D.A., Watson, R.J., Biessen, E.A., Chapman, G.A., van Berkel, T.J.: CXCR3 antagonist NBI-74330 attenuates atherosclerotic plaque formation in LDL receptor–deficient mice. Arterioscler. Thromb. Vasc. Biol. 28(2), 251–257 (2008)
Combadière, C., Potteaux, S., Rodero, M., Simon, T., Pezard, A., Esposito, B., Merval, R., Proudfoot, A., Tedgui, A., Mallat, Z.: Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6Chi and Ly6Clo monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation 117(13), 1649–1657 (2008)
Veillard, N.R., Steffens, S., Pelli, G., Lu, B., Kwak, B.R., Gerard, C., Charo, I.F., Mach, F.: Differential influence of chemokine receptors CCR2 and CXCR3 in development of atherosclerosis in vivo. Circulation 112(6), 870–878 (2005)
Potteaux, S., Combadiere, C., Esposito, B., Lecureuil, C., Ait-Oufella, H., Merval, R., Ardouin, P., Tedgui, A., Mallat, Z.: Role of bone marrow-derived CC-chemokine receptor 5 in the development of atherosclerosis of low-density lipoprotein receptor knockout mice. Arterioscler. Thromb. Vasc. Biol. 26(8), 1858–1863 (2006)
Braunersreuther, V., Zernecke, A., Arnaud, C., Liehn, E.A., Steffens, S., Shagdarsuren, E., Bidzhekov, K., Burger, F., Pelli, G., Luckow, B.: Ccr5 but not Ccr1 deficiency reduces development of diet-induced atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 27(2), 373–379 (2007)
Lesnik, P., Haskell, C.A., Charo, I.F.: Decreased atherosclerosis in CX 3 CR1−/−mice reveals a role for fractalkine in atherogenesis. J. Clin. Invest. 111(3), 333–340 (2003)
Combadière, C., Potteaux, S., Gao, J.-L., Esposito, B., Casanova, S., Lee, E.J., Debré, P., Tedgui, A., Murphy, P.M., Mallat, Z.: Decreased atherosclerotic lesion formation in CX3CR1/apolipoprotein E double knockout mice. Circulation 107(7), 1009–1016 (2003)
Liu, P., Yen-Rei, A.Y., Spencer, J.A., Johnson, A.E., Vallanat, C.T., Fong, A.M., Patterson, C., Patel, D.D.: CX3CR1 deficiency impairs dendritic cell accumulation in arterial intima and reduces atherosclerotic burden. Arterioscler. Thromb. Vasc. Biol. 28(2), 243–250 (2008)
Teupser, D., Pavlides, S., Tan, M., Gutierrez-Ramos, J.-C., Kolbeck, R., Breslow, J.L.: Major reduction of atherosclerosis in fractalkine (CX3CL1)-deficient mice is at the brachiocephalic artery, not the aortic root. Proc. Natl. Acad. Sci. U. S. A. 101(51), 17795–17800 (2004)
Nageh, M.F., Sandberg, E.T., Marotti, K.R., Lin, A.H., Melchior, E.P., Bullard, D.C., Beaudet, A.L.: Deficiency of inflammatory cell adhesion molecules protects against atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 17(8), 1517–1520 (1997)
Shih, P.T., Brennan, M.-L., Vora, D.K., Territo, M.C., Strahl, D., Elices, M.J., Lusis, A.J., Berliner, J.A.: Blocking very late antigen-4 integrin decreases leukocyte entry and fatty streak formation in mice fed an atherogenic diet. Circ. Res. 84(3), 345–351 (1999)
Lowe, J.B.: Glycosylation in the control of selectin counter‐receptor structure and function. Immunol. Rev. 186(1), 19–36 (2002)
Frommhold, D., Ludwig, A., Bixel, M.G., Zarbock, A., Babushkina, I., Weissinger, M., Cauwenberghs, S., Ellies, L.G., Marth, J.D., Beck-Sickinger, A.G., Sixt, M., Lange-Sperandio, B., Zernecke, A., Brandt, E., Weber, C., Vestweber, D., Ley, K., Sperandio, M.: Sialyltransferase ST3Gal-IV controls CXCR2-mediated firm leukocyte arrest during inflammation. J. Exp. Med. 205(6), 1435–1446 (2008)
Sperandio, M.: Selectins and glycosyltransferases in leukocyte rolling in vivo. Febs J. 273(19), 4377–4389 (2006)
Bannert, N., Craig, S., Farzan, M., Sogah, D., Santo, N.V., Choe, H., Sodroski, J.: Sialylated O-glycans and sulfated tyrosines in the NH2-terminal domain of CC chemokine receptor 5 contribute to high affinity binding of chemokines. J. Exp. Med. 194(11), 1661–1673 (2001)
Harduin-Lepers, A., Vallejo-Ruiz, V., Krzewinski-Recchi, M.A., Samyn-Petit, B., Julien, S., Delannoy, P.: The human sialyltransferase family. Biochimie 83(8), 727–737 (2001)
Doring, Y., Noels, H., Mandl, M., Kramp, B., Neideck, C., Lievens, D., Drechsler, M., Megens, R.T., Tilstam, P.V., Langer, M., Hartwig, H., Theelen, W., Marth, J.D., Sperandio, M., Soehnlein, O., Weber, C.: Deficiency of the sialyltransferase St3Gal4 reduces Ccl5-mediated myeloid cell recruitment and arrest: short communication. Circ. Res. 114(6), 976–981 (2014)
Sperandio, M., Frommhold, D., Babushkina, I., Ellies, L.G., Olson, T.S., Smith, M.L., Fritzsching, B., Pauly, E., Smith, D.F., Nobiling, R., Linderkamp, O., Marth, J.D., Ley, K.: Alpha 2,3-sialyltransferase-IV is essential for L-selectin ligand function in inflammation. Eur. J. Immunol. 36(12), 3207–3215 (2006)
Homeister, J.W., Daugherty, A., Lowe, J.B.: Alpha(1,3)fucosyltransferases FucT-IV and FucT-VII control susceptibility to atherosclerosis in apolipoprotein E−/− mice. Arterioscler. Thromb. Vasc. Biol. 24(10), 1897–1903 (2004)
Gitlin, J.M., Homeister, J.W., Bulgrien, J., Counselman, J., Curtiss, L.K., Lowe, J.B., Boisvert, W.A.: Disruption of tissue-specific fucosyltransferase VII, an enzyme necessary for selectin ligand synthesis, suppresses atherosclerosis in mice. Am. J. Pathol. 174(1), 343–350 (2009)
Malý, P., Thall, A.D., Petryniak, B., Rogers, C.E., Smith, P.L., Marks, R.M., Kelly, R.J., Gersten, K.M., Cheng, G., Saunders, T.L., Camper, S.A., Camphausen, R.T., Sullivan, F.X., Isogai, Y., Hindsgaul, O., von Andrian, U.H., Lowe, J.B.: The α(1,3)Fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell 86(4), 643–653 (1996)
Homeister, J.W., Thall, A.D., Petryniak, B., Maly, P., Rogers, C.E., Smith, P.L., Kelly, R.J., Gersten, K.M., Askari, S.W., Cheng, G., Smithson, G., Marks, R.M., Misra, A.K., Hindsgaul, O., von Andrian, U.H., Lowe, J.B.: The alpha(1,3)fucosyltransferases FucT-IV and FucT-VII exert collaborative control over selectin-dependent leukocyte recruitment and lymphocyte homing. Immunity 15(1), 115–126 (2001)
Li, F., Wilkins, P.P., Crawley, S., Weinstein, J., Cummings, R.D., McEver, R.P.: Post-translational modifications of recombinant P-selectin glycoprotein ligand-1 required for binding to P-and E-selectin. J. Biol. Chem. 271(6), 3255–3264 (1996)
Wang, H., Tang, R., Zhang, W., Amirikian, K., Geng, Z., Geng, J., Hebbel, R.P., Xia, L., Marth, J.D., Fukuda, M.: Core2 1-6-N-Glucosaminyltransferase-I is crucial for the formation of atherosclerotic lesions in apolipoprotein e–deficient mice. Arterioscler. Thromb. Vasc. Biol. 29(2), 180–187 (2009)
Barran, P., Fellinger, W., Warren, C.E., Dennis, J.W., Ziltener, H.J.: Modification of CD43 and other lymphocyte O-glycoproteins by core 2 N-acetylglucosaminyltransferase. Glycobiology 7(1), 129–136 (1997)
Carlow, D.A., Ziltener, H.J.: CD43 deficiency has no impact in competitive in vivo assays of neutrophil or activated T cell recruitment efficiency. J. Immunol. (Baltimore, Md. : 1950) 177(9), 6450–6459 (2006)
Kumar, R., Camphausen, R.T., Sullivan, F.X., Cumming, D.A.: Core2 beta-1,6-N-acetylglucosaminyltransferase enzyme activity is critical for P-selectin glycoprotein ligand-1 binding to P-selectin. Blood 88(10), 3872–3879 (1996)
Hadzibegovic, I., Vrselja, Z., Lauc, G., Curic, G.: Expression of leukocyte adhesion-related glycosyltransferase genes in acute coronary syndrome patients. Inflamm. Res. 63(8), 629–636 (2014)
Cullen, P., Mohr, S., Brennhausen, B., Cignarella, A., Assmann, G.: Downregulation of the selectin ligand-producing fucosyltransferases Fuc-TIV and Fuc-TVII during foam cell formation in monocyte-derived macrophages. Arterioscler. Thromb. Vasc. Biol. 17(8), 1591–1598 (1997)
Acknowledgments
This work was supported by the National Nature Science Foundation of China (Project no. 81370403) and Specialized Research Fund for the Doctoral Program of Higher Education (No.20125503110008).
Conflict of interest
The authors have declared no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pu, Q., Yu, C. Glycosyltransferases, glycosylation and atherosclerosis. Glycoconj J 31, 605–611 (2014). https://doi.org/10.1007/s10719-014-9560-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-014-9560-8