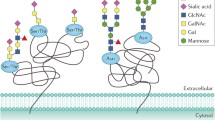
Abstract
Altered glycosylation is a universal feature of cancer cells and altered glycans can help cancer cells escape immune surveillance, facilitate tumor invasion, and increase malignancy. The goal of this study was to identify specific glycoenzymes, which could distinguish prostate cancer cells from normal prostatic cells. We investigated enzymatic activities and gene expression levels of key glycosyl- and sulfotransferases responsible for the assembly of O- and N-glycans in several prostatic cells. These cells included immortalized RWPE-1 cells derived from normal prostatic tissues, and prostate cancer cells derived from metastasis in bone (PC-3), brain (DU145), lymph node (LNCaP), and vertebra (VCaP). We found that all cells were capable of synthesizing complex N-glycans and O-glycans with the core 1 structure, and each cell line had characteristic biosynthetic pathways to modify these structures. The in vitro measured activities corresponded well to the mRNA levels of glycosyltransferases and sulfotransferases. Lectin and antibody binding to whole cells supported these results, which form the basis for the development of tumor cell-specific targeting strategies.



Similar content being viewed by others
Abbreviations
- FUT:
-
fucosyltransferase
- Gal3ST:
-
3-O-sulfotransferase
- GalT:
-
galactosyltransferase
- GalNAcT:
-
GalNAc-transferase
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- GnT GlcNAcT:
-
GlcNAc-transferase
- GlcNAc6ST:
-
N-acetylglucosaminyl-6-O-sulfotransferase
- HPLC:
-
high pressure liquid chromatography
- PCR:
-
polymerase chain reaction
- ppGalNAcT:
-
polypeptide GalNAc-transferase
- PSA:
-
prostate specific antigen
- SLex :
-
sialyl-Lewisx
- ST3Gal:
-
α2,3-sialyltransferase
- ST6Gal(NAc):
-
α2,6-sialyltransferase
References
Bresalier, R.S., Ho, S.B., Schoeppner, H.L., Kim, Y.S., Sleisenger, M.H., Brodt, P., Byrd, J.C.: Enhanced sialylation of mucin-associated carbohydrate structures in human colon cancer metastasis. Gastroenterology 110, 1354–1367 (1996)
Burke, P.A., Gregg, J.P., Bakhtiar, B., Beckett, L.A., Denardo, G.L., Albrecht, H., De Vere White, R.W., De Nardo, S.J.: Characterization of MUC1 glycoprotein on prostate cancer for selection of targeting molecules. Int. J. Oncol. 29, 49–55 (2006)
Garbar, C., Mascaux, C., Wespes, E.: Expression of MUC1 and sialyl-Tn in benign prostatic glands, high-grade prostate intraepithelial neoplasia and malignant prostatic glands: a preliminary study. Anal. Quant. Cytol. Histol. 30, 71–77 (2008)
Tabarés, G., Radcliffe, C.M., Barrabés, S., Ramírez, M., Aleixandre, R.N., Hoesel, W., Dwek, R.A., Rudd, P.M., Peracaula, R., de Llorens, R.: Different glycan structures in prostate-specific antigen from prostate cancer sera in relation to seminal plasma PSA. Glycobiology 16, 132–145 (2006)
Barthel, S.R., Gavino, J.D., Wiese, G.K., Jaynes, J.M., Siddiqui, J., Dimitroff, C.J.: Analysis of glycosyltransferase expression in metastatic prostate cancer cells capable of rolling activity on microvascular endothelial (E)-selectin. Glycobiology 18, 806–817 (2008)
Barthel, S.R., Wiese, G.K., Cho, J., Opperman, M.J., Hays, D.L., Siddiqui, J., Pienta, K.J., Furie, B., Dimitroff, C.J.: Alpha 1,3 fucosyltransferases are master regulators of prostate cancer cell trafficking. Proc. Natl. Acad. Sci. U. S. A. 106, 19491–19496 (2009)
Brockhausen, I.: Mucin-type O-glycans in human colon and breast cancer: glycodynamics and functions. EMBO Rep. 7, 599–604 (2006)
Carraway, K.L., Fregien, N., Carraway, C.A.: Tumor sialomucin complexes as tumor antigens and modulators of cellular interactions and proliferation. J. Cell Sci. 103(Pt 2), 299–307 (1992)
David, L., Nesland, J.M., Clausen, H., Carneiro, F., Sobrinho-Simões, M.: Simple mucin-type carbohydrate antigens (Tn, sialosyl-Tn and T) in gastric mucosa, carcinomas and metastases. APMIS Suppl. 27, 162–172 (1992)
Hoff, S.D., Matsushita, Y., Ota, D.M., Cleary, K.R., Yamori, T., Hakomori, S., Irimura, T.: Increased expression of sialyl-dimeric LeX antigen in liver metastases of human colorectal carcinoma. Cancer Res. 49, 6883–6888 (1989)
Kojima, N., Handa, K., Newman, W., Hakomori, S.: Inhibition of selectin-dependent tumor cell adhesion to endothelial cells and platelets by blocking O-glycosylation of these cells. Biochem. Biophys. Res. Commun. 182, 1288–1295 (1992)
Radhakrishnan, P., Lin, M.F., Cheng, P.W.: Elevated expression of L-selectin ligand in lymph node-derived human prostate cancer cells correlates with increased tumorigenicity. Glycoconj. J. 26, 75–81 (2009)
Takano, R., Muchmore, E., Dennis, J.W.: Sialylation and malignant potential in tumour cell glycosylation mutants. Glycobiology 4, 665–674 (1994)
Itzkowitz, S.H., Bloom, E.J., Kokal, W.A., Modin, G., Hakomori, S., Kim, Y.S.: Sialosyl-Tn. A novel mucin antigen associated with prognosis in colorectal cancer patients. Cancer 66, 1960–1966 (1990)
Janković, M.M., Kosanović, M.M.: Glycosylation of urinary prostate-specific antigen in benign hyperplasia and cancer: assessment by lectin-binding patterns. Clin. Biochem. 38, 58–65 (2005)
Peracaula, R., Tabarés, G., Royle, L., Harvey, D.J., Dwek, R.A., Rudd, P.M., de Llorens, R.: Altered glycosylation pattern allows the distinction between prostate-specific antigen (PSA) from normal and tumor origins. Glycobiology 13, 457–470 (2003)
Cozzi, P.J., Wang, J., Delprado, W., Perkins, A.C., Allen, B.J., Russell, P.J., Li, Y.: MUC1, MUC2, MUC4, MUC5AC and MUC6 expression in the progression of prostate cancer. Clin. Exp. Metastasis 22, 565–573 (2005)
Singh, A.P., Chauhan, S.C., Bafna, S., Johansson, S.L., Smith, L.M., Moniaux, N., Lin, M.F., Batra, S.K.: Aberrant expression of transmembrane mucins, MUC1 and MUC4, in human prostate carcinomas. Prostate 66, 421–429 (2006)
Wu, G.J., Peng, Q., Fu, P., Wang, S.W., Chiang, C.F., Dillehay, D.L., Wu, M.W.: Ectopical expression of human MUC18 increases metastasis of human prostate cancer cells. Gene 327, 201–213 (2004)
Bélanger, A., van Halbeek, H., Graves, H.C., Grandbois, K., Stamey, T.A., Huang, L., Poppe, I., Labrie, F.: Molecular mass and carbohydrate structure of prostate specific antigen: studies for establishment of an international PSA standard. Prostate 27, 187–197 (1995)
Meany, D.L., Zhang, Z., Sokoll, L.J., Zhang, H., Chan, D.W.: Glycoproteomics for prostate cancer detection: changes in serum PSA glycosylation patterns. J. Proteome Res. 8, 613–619 (2009)
Ohyama, C., Hosono, M., Nitta, K., Oh-eda, M., Yoshikawa, K., Habuchi, T., Arai, Y., Fukuda, M.: Carbohydrate structure and differential binding of prostate specific antigen to Maackia amurensis lectin between prostate cancer and benign prostate hypertrophy. Glycobiology 14, 671–679 (2004)
St Hill, C.A., Farooqui, M., Mitcheltree, G., Gulbahce, H.E., Jessurun, J., Cao, Q., Walcheck, B.: The high affinity selectin glycan ligand C2-O-SLex and mRNA transcripts of the core 2 beta-1,6-N-acetylglucosaminyltransferase (C2GnT1) gene are highly expressed in human colorectal adenocarcinomas. BMC Cancer 9, 79 (2009)
Shimodaira, K., Nakayama, J., Nakamura, N., Hasebe, O., Katsuyama, T., Fukuda, M.: Carcinoma-associated expression of core 2 beta-1,6-N-acetylglucosaminyltransferase gene in human colorectal cancer: role of O-glycans in tumor progression. Cancer Res. 57, 5201–5206 (1997)
Hanski, C., Klussmann, E., Wang, J., Böhm, C., Ogorek, D., Hanski, M.L., Krüger-Krasagakes, S., Eberle, J., Schmitt-Gräff, A., Riecken, E.O.: Fucosyltransferase III and sialyl-Le(x) expression correlate in cultured colon carcinoma cells but not in colon carcinoma tissue. Glycoconj. J. 13, 727–733 (1996)
Ito, H., Hiraiwa, N., Sawada-Kasugai, M., Akamatsu, S., Tachikawa, T., Kasai, Y., Akiyama, S., Ito, K., Takagi, H., Kannagi, R.: Altered mRNA expression of specific molecular species of fucosyl- and sialyl-transferases in human colorectal cancer tissues. Int. J. Cancer 71, 556–564 (1997)
Nakamori, S., Kameyama, M., Imaoka, S., Furukawa, H., Ishikawa, O., Sasaki, Y., Kabuto, T., Iwanaga, T., Matsushita, Y., Irimura, T.: Increased expression of sialyl Lewisx antigen correlates with poor survival in patients with colorectal carcinoma: clinicopathological and immunohistochemical study. Cancer Res. 53, 3632–3637 (1993)
Machida, E., Nakayama, J., Amano, J., Fukuda, M.: Clinicopathological significance of core 2 beta1,6-N-acetylglucosaminyltransferase messenger RNA expressed in the pulmonary adenocarcinoma determined by in situ hybridization. Cancer Res. 61, 2226–2231 (2001)
Hagisawa, S., Ohyama, C., Takahashi, T., Endoh, M., Moriya, T., Nakayama, J., Arai, Y., Fukuda, M.: Expression of core 2 beta1,6-N-acetylglucosaminyltransferase facilitates prostate cancer progression. Glycobiology 15, 1016–1024 (2005)
Valenzuela, H.F., Pace, K.E., Cabrera, P.V., White, R., Porvari, K., Kaija, H., Vihko, P., Baum, L.G.: O-glycosylation regulates LNCaP prostate cancer cell susceptibility to apoptosis induced by galectin-1. Cancer Res. 67, 6155–6162 (2007)
Brockhausen, I.: Pathways of O-glycan biosynthesis in cancer cells. Biochim. Biophys. Acta 1473, 67–95 (1999)
Brockhausen, I.: Comprehensive Natural Products II Chemistry and Biology. In: Mander, L., Lui, H.-W., Wang, P.G. (eds.) Biosynthesis of complex mucin-type O-glycans. Carbohydrates, nucleosides and nucleic acids, vol. 6, pp. 315–350. Elsevier, Oxford (2010). Chapter 11
Burchell, J., Poulsom, R., Hanby, A., Whitehouse, C., Cooper, L., Clausen, H., Miles, D., Taylor-Papadimitriou, J.: An alpha2,3 sialyltransferase (ST3Gal I) is elevated in primary breast carcinomas. Glycobiology 9, 1307–1311 (1999)
Petretti, T., Kemmner, W., Schulze, B., Schlag, P.M.: Altered mRNA expression of glycosyltransferases in human colorectal carcinomas and liver metastases. Gut 46, 359–366 (2000)
Seko, A., Ohkura, T., Kitamura, H., Yonezawa, S., Sato, E., Yamashita, K.: Quantitative differences in GlcNAc:beta1– > 3 and GlcNAc:beta1– > 4 galactosyltransferase activities between human colonic adenocarcinomas and normal colonic mucosa. Cancer Res. 56, 3468–3473 (1996)
Yang, J.M., Byrd, J.C., Siddiki, B.B., Chung, Y.S., Okuno, M., Sowa, M., Kim, Y.S., Matta, K.L., Brockhausen, I.: Alterations of O-glycan biosynthesis in human colon cancer tissues. Glycobiology 4, 873–884 (1994)
Vavasseur, F., Dole, K., Yang, J., Matta, K.L., Myerscough, N., Corfield, A., Paraskeva, C., Brockhausen, I.: O-glycan biosynthesis in human colorectal adenoma cells during progression to cancer. Eur. J. Biochem. 222, 415–424 (1994)
Vavasseur, F., Yang, J.M., Dole, K., Paulsen, H., Brockhausen, I.: Synthesis of O-glycan core 3: characterization of UDP-GlcNAc: GalNAc-R beta 3-N-acetyl-glucosaminyltransferase activity from colonic mucosal tissues and lack of the activity in human cancer cell lines. Glycobiology 5, 351–357 (1995)
Brockhausen, I., Romero, P., Herscovics, A.: Glycosyltransferase changes upon differentiation of CaCo-2 human colonic adenocarcinoma Cells. Cancer Res. 51, 3136–3142 (1991)
Brockhausen, I., Benn, M., Bhat, S., Marone, S., Riley, J.G., Montoya-Peleaz, P., Vlahakis, J.Z., Paulsen, H., Schutzbach, J.S., Szarek, W.A.: UDP-Gal: GlcNAc-R beta1,4-galactosyltransferase–a target enzyme for drug design. Acceptor specificity and inhibition of the enzyme. Glycoconj. J. 23, 525–541 (2006)
Brockhausen, I., Reck, F., Kuhns, W., Khan, S., Matta, K.L., Meinjohanns, E., Paulsen, H., Shah, R.N., Baker, M.A., Schachter, H.: Substrate specificity and inhibition of UDP-GlcNAc:GlcNAc beta 1-2Man alpha 1-6R beta 1,6-N-acetylglucosaminyltransferase V using synthetic substrate analogues. Glycoconj. J. 12, 371–379 (1995)
Brockhausen, I., Dowler, T., Paulsen, H.: Site directed processing: role of amino acid sequences and glycosylation of acceptor glycopeptides in the assembly of extended mucin type O-glycan core 2. Biochim. Biophys. Acta 1790, 1244–1257 (2009)
Tassone, F., Hagerman, R.J., Taylor, A.K., Gane, L.W., Godfrey, T.E., Hagerman, P.J.: Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome. Am. J. Hum. Genet. 66, 6–15 (2000)
Yang, X., Yip, J., Harrison, M., Brockhausen, I.: Primary human osteoblasts and bone cancer cells as models to study glycodynamics in bone. Int. J. Biochem. Cell Biol. 40, 471–483 (2008)
Brown, J.R., Fuster, M.M., Li, R., Varki, N., Glass, C.A., Esko, J.D.: A disaccharide-based inhibitor of glycosylation attenuates metastatic tumor cell dissemination. Clin. Cancer Res. 12, 2894–2901 (2006)
Brockhausen, I., Williams, D., Matta, K.L., Orr, J., Schachter, H.: Mucin Synthesis III: UDP-GlcNAc:Galβ1-3(GlcNAcβ1-6)GalNAc-R (GlcNAc to Gal) β3-N-acetylglucosaminyltransferase, an enzyme in porcine gastric mucosa involved in the elongation of mucin-type oligosaccharides. Can. J. Biochem. Cell Biol. 61, 1322–1333 (1983)
Gao, Y., Lazar, C., Szarek, W.A., Brockhausen, I.: Specificity of β4galactosyltransferase inhibitor 2-naphthyl 2-butanamido-2-deoxy-1-thio-β-D-glucopyranoside. Glycoconj. J. 27, 673–684 (2010)
Huang, J., Liang, J.T., Huang, H.C., Shen, T.L., Chen, H.Y., Lin, N.Y., Che, M.I., Lin, W.C., Huang, M.C.: Beta1,4-N-acetylgalactosaminyltransferase III enhances malignant phenotypes of colon cancer cells. Mol. Cancer Res. 5, 543–552 (2007)
Yao, M., Zhou, D.P., Jiang, S.M., Wang, Q.H., Zhou, X.D., Tang, Z.Y., Gu, J.X.: Elevated activity of N-acetylglucosaminyltransferase V in human hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 124, 27–30 (1998)
Tsui, K.H., Chang, P.L., Feng, T.H., Chung, L.C., Sung, H.C., Juang, H.H.: Evaluating the function of matriptase and N-acetylglucosaminyltransferase V in prostate cancer metastasis. Anticancer. Res. 28, 1993–1999 (2008)
Premaratne, P., Wélen, K., Damber, J.-E., Hansson, G., Bäckström, M.: O-glycosylation of MUC1 mucin in prostate cancer and the effects of its expression on tumor growth in a prostate cancer xenograft model. Tumor Biol. 32, 203–213 (2011)
Pinho, S., Marcos, N.T., Ferreira, B., Carvalho, A.S., Oliveira, M.J., Santos-Silva, F., Harduin-Lepers, A., Reis, C.A.: Biological significance of cancer-associated sialyl-Tn antigen: modulation of malignant phenotype in gastric carcinoma cells. Cancer Lett. 249, 157–170 (2007)
Lise, M., Belluco, C., Perera, S.P., Patel, R., Thomas, P., Ganguly, A.: Clinical correlations of alpha2,6-sialyltransferase expression in colorectal cancer patients. Hybridoma 19, 281–286 (2000)
Dalziel, M., Whitehouse, C., McFarlane, I., Brockhausen, I., Gschmeissner, S., Schwientek, T., Clausen, H., Burchell, J.M., Taylor-Papadimitriou, J.: The relative activities of the C2GnT1 and ST3Gal-I glycosyltransferases determine O-glycan structure and expression of a tumor-associated epitope on MUC1. J. Biol. Chem. 276, 11007–11015 (2001)
Hatakeyama, S., Kyan, A., Yamamoto, H., Okamoto, A., Sugiyama, N., Suzuki, Y., Yoneyama, T., Hashimoto, Y., Koie, T., Yamada, S., Saito, H., Arai, Y., Fukuda, M., Ohyama, C.: Core 2 N-acetylglucosaminyltransferase-1 expression induces aggressive potential of testicular germ cell tumor. Int. J. Cancer 127, 1052–1059 (2010)
Iwai, T., Kudo, T., Kawamoto, R., Kubota, T., Togayachi, A., Hiruma, T., Okada, T., Kawamoto, T., Morozumi, K., Narimatsu, H.: Core 3 synthase is down-regulated in colon carcinoma and profoundly suppresses the metastatic potential of carcinoma cells. Proc. Natl. Acad. Sci. U. S. A. 102, 4572–4577 (2005)
Lee, S.H., Hatakeyama, S., Yu, S.Y., Bao, X., Ohyama, C., Khoo, K.H., Fukuda, M.N., Fukuda, M.: Core 3 O-glycan synthase suppresses tumor formation and metastasis of prostate carcinoma PC3 and LNCaP cells through down-regulation of alpha2beta1 integrin complex. J. Biol. Chem. 284, 17157–17169 (2009)
An, G., Wei, B., Xia, B., McDaniel, J.M., Ju, T., Cummings, R.D., Braun, J., Xia, L.: Increased susceptibility to colitis and colorectal tumors in mice lacking core 3-derived O-glycans. J. Exp. Med. 240, 1417–1429 (2007)
Brockhausen, I., Yang, J.M., Burchell, J., Whitehouse, C., Taylor-Papadimitriou, J.: Mechanisms underlying aberrant glycosylation of MUC1 mucin in breast cancer cells. Eur. J. Biochem. 233, 607–617 (1995)
Cazet, A., Julien, S., Bobowski, M., Krzewinski-Recchi, M.A., Harduin-Lepers, A., Groux-Degroote, S., Delannoy, P.: Consequences of the expression of sialylated antigens in breast cancer. Carbohydr. Res. 345, 1377–1383 (2010)
Mungul, A., Cooper, L., Brockhausen, I., Ryder, K., Mandel, U., Clausen, H., Rughetti, A., Miles, D.W., Taylor-Papadimitriou, J., Burchell, J.M.: Sialylated core 1 based O-linked glycans enhance the growth rate of mammary carcinoma cells in MUC1 transgenic mice. Int. J. Oncol. 25, 937–943 (2004)
Picco, G., Julien, S., Brockhausen, I., Beatson, R., Antonopoulos, A., Haslam, S., Mandel, U., Dell, A., Pinder, S., Taylor-Papadimitriou, J., Burchell, J.: Over-expression of ST3Gal-I promotes mammary tumorigenesis. Glycobiology 20, 1241–1250 (2010)
Acknowledgements
This work was supported by a grant from the Prostate Cancer Fight Foundation, Motorcycle Ride for Dad (to I.B.), and grants from the Office of Research and Development, Medical Research Service, Department of Veterans Affairs (VA 1I1BX000985), the National Institutes of Health (1R21HL097238 and 2RO1HL48282) and the State of Nebraska (LB506)(to P.W.C.).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
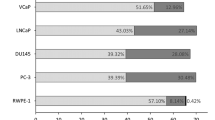
Lectin staining of whole prostatic cells. Lectin binding was carried out as described in the Methods section using Ricin (Ricin A chain lectin), WGA (wheat germ agglutinin), MAAII (Maackia amurensis lectin II), SNA (Sambucus nigra lectin), GSL-1 (Griffonia simplicifolia lectin 1), UEA-1 (Ulex eauropaeus lectin 1), PNA (Peanut agglutinin), HP, (Helix pomatia lectin), ConA (Concanavalin A), BSL-1 (Bandeiraea simplicifolia lectin 1), DSL ( Datura stramonium lectin). The intensity of absorbance at 405 nm was recorded, normalized to the same cell number of 100,000 cells (Intensity). a, Normal prostatic RWPE-1 cells; b, Prostate cancer cells PC-3; c, DU145 cells; d, LNCaP cells; e, VCaP cells. The error bars show the variations among the 7 samples tested. (PDF 223 kb)
Supplementary Figure 2
ELISA of whole prostatic cells. Prostatic cells were stained as described in the Materials and Methods section using anti-Tn antibody (Tn) anti-sialyl-Tn (STn), anti-sialyl-Lewisx (SLe x), anti- Lewis y (Le y) and anti-Lewis a (Le a) antibodies. The intensity of staining measured at 405 nm , normalized to the same cell number is shown. a, Normal prostatic RWPE-1 cells; b, Prostate cancer cells PC-3; c, DU145 cells; d, LNCaP cells; e, VCaP cells. (PDF 100 kb)
Supplementary Figure 3
Quantitative real time PCR analysis of various glycosyltransferase and sulfotransferase genes in normal and cancerous prostatic cells. The expression levels of glycosyltransferase and sulfotransferase genes are shown. The gene expression levels were calculated by the ΔCt method as described in Materials and Methods and expressed Supplemental Material (Not to be Published) as relative amount to that of GAPDH (100%). The enzyme names are listed in Table 1. Results are shown for B3GNT2 - 5 (Extension β3GlcNAcT), ST3GAL3 - 6 (α2,3-sialyltransferases), GAL3ST1 and 3 (galactosyl-3-O-sulfotransferases); ST6GALNAC1 - 4 (α2,6-sialyltransferases acting on GalNAc) and ST6GAL1 (α2,6-sialyltransferase acting on Gal). The data were obtained from three independent experiments and expressed as mean ± SEM. (PDF 181 kb)
ESM 1
(PDF 37 kb)
Rights and permissions
About this article
Cite this article
Gao, Y., Chachadi, V.B., Cheng, PW. et al. Glycosylation potential of human prostate cancer cell lines. Glycoconj J 29, 525–537 (2012). https://doi.org/10.1007/s10719-012-9428-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-012-9428-8