Abstract
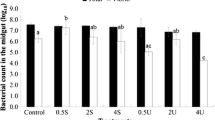
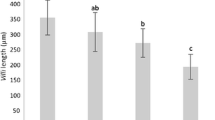
Larval rearing is affected by a wide range of microorganisms that thrive in larviculture systems. Some seaweed species have metabolites capable of reducing the bacterial load. However, no studies have yet tested whether including seaweed metabolites on larval rearing systems has any effects on the larvae development. This work assessed the development of Sparus aurata larvae fed preys treated with an Asparagopsis armata product. Live prey, Brachionus spp. and Artemia sp., were immersed in a solution containing 0.5% of a commercial extract of A. armata (Ysaline 100, YSA) for 30 min, before being fed to seabream larvae (n = 4 each). In the control, the live feed was immersed in clear water. Larval parameters such as growth, survival, digestive capacity (structural-histology and functional-enzymatic activity), stress level (cortisol content), non-specific immune response (lysozyme activity), anti-bacterial activity (disc-diffusion assay) and microbiota quantification (fish larvae gut and rearing water) were monitored. Fish larvae digestive capacity, stress level and non-specific immune response were not affected by the use of YSA. The number of Vibrionaceae was significantly reduced both in water and larval gut when using YSA. Growth was enhanced for YSA treatment, but higher mortality was also observed, especially until 10 days after hatching (DAH). The mortality peak observed at 8 DAH for both treatments, but higher for YSA, indicates larval higher susceptibility at this development stage, suggesting that lower concentrations of YSA should be used until 10 DAH. The application of YSA after 10 DAH onwards promotes a safer rearing environment.



Similar content being viewed by others
References
Anderson DP, Zeeman MG (1995) Immunotoxicology in fish. In: Rand GM (ed) Fundamentals of aquatic toxicology, 2nd edn. Taylor and Francis, London, pp 371–402
Attramadal K, Øie G, Størseth T, Alver M, Vadstein O, Olsen Y (2012) The effects of moderate ozonation or high intensive UV-irradiation on the microbial environment in RAS for marine larvae. Aquaculture 300(333):121–129
Bansemir A, Bleme M, Schröder S, Lindequist U (2006) Screening of cultivated seaweeds for antibacterial activity against fish pathogenic bacteria. Aquaculture 252:79–84
Barton BA (2002) Stress in fishes: a diversity of responses with particular reference to changes in circulating corticosteroids. Integ and Comp Biol 42:517–525
Bessey OA, Lowry OH, Brock MJ (1946) A method for the rapid determination of alkaline phosphates with five cubic millimetres of serum. J Biol Chem 164:321–329
Bradford MM (1976) A rapid sensitive method for the quantification of microgram quantities of protein utilising the principle of protein-dye binding. Anal Biochem 72:248–254
Bricknell I, Dalmo RA (2005) The use of immunostimulants in fish larval aquaculture. Fish Shellfish Immunol 19:457–472
Califano G, Castanho S, Soares F, Ribeiro L, Cox CJ, Mata L, Costa R (2017) Molecular taxonomic profiling of bacterial communities in a gilthead seabream (Sparus aurata) hatchery. Front Microbiol 8:204. doi:10.3389/fmicb.2017.00204
Cañavate JP, Fernández-Díaz C (2001) Pilot evaluation of freeze-dried microalgae in the mass rearing of gilthead seabream (Sparus aurata) larvae. Aquaculture 193:257–269
Cecchini S, Terova G, Caricato G, Saroglia M (2000) Lysozyme activity in embryos and larvae of sea bass (Dicentrarchus labrax L.), spawned by broodstocks fed with vitamin C enriched diets. Bull Eur Ass Fish Pathol 20(3):120–124
Chabbert YA (1963) L’antibiogramme. Sensibilité et résistance des bactéries aux antibiotiques. Edited by St-Mandé, Editions de la Tourelle
Citarasu T (2010) Herbal biomedicines: a new opportunity for aquaculture industry. Aquac Int 18:403–414
Colloca F, Cerasi S (2005) Cultured aquatic species information programme, Sparus aurata. FAO Fisheries and Aquaculture Department. Rome. www.fao.org/fishery/culturedspecies/Sparus_aurata/en. Accessed September 2013
Conceição LEC, Yúfera M, Makridis P, Morais S, Dinis MT (2010) Live feeds for early stages of fish rearing. Aquac Res 41:613–640
Dhert P, Rombaut G, Suantika G, Sorgeloos P (2001) Advancement of rotifer culture and manipulation techniques in Europe. Aquaculture 200:129–146
Dimitroglou A, Merrifield D, Carnevali O, Picchietti S, Avella M, Daniels C, Güroy D, Davies SJ (2011) Microbial manipulations to improve fish health and production – a Mediterranean perspective. Fish Shellfish Immunol 30:1–16
Elbal MT, García Hernández MP, Lozano MT, Agulleiro B (2004) Development of the digestive tract of gilthead seabream (Sparus aurata L.). Light and electron microscopic studies. Aquaculture 234:215–238
EPA (1989) Health and environmental effects document for bromoform. Cincinnati.
FAO (2012) The state of world fisheries and aquaculture. Rome.
Gisbert E, Ortiz-Delgado JB, Sarasquete C (2008) Nutritional cellular biomarkers in early life stages of fish. Histol Histopathol 23:1525–1539
Haché R, Plante S (2011) The relationship between enrichment, fatty acid profiles and bacterial load in cultured rotifers (Brachionus plicatilis L-strain) and Artemia (Artemia salina strain Franciscana). Aquaculture 311:201–208
Hamlin HJ, von Herbing IH, Kling LJ (2000) Histological and morphological evaluations of the digestive tract and associated organs of haddock throughout post-hatching ontogeny. J Fish Biol 57:716–732
Harikrishnan R, Balasundaram C, Heo MS (2011) Impact of plant products on innate and adaptive immune system of cultured finfish and shellfish. Aquaculture 317:1–15
Kjorsvik E, Galloway TF, Estevez A, Sӕle Ø, Moren M (2011) Effects of larval nutrition on development In: Holt, GJ (ed). Larval Fish Nutrition. Wiley-Blackewll, pp 219–248
Lazo JP, Darias MJ, Gisbert E (2011) Ontogeny of the digestive tract in larval. In: Holt GJ, (ed) Fish Nutrition. Wiley-Blackwell, pp 5–46
Magariños B, Pazos F, Santos Y, Romalde JL, Toranzo AE (1995) Response of Pasteurella piscicida and Flexibacter maritimus to skin mucus of marine fish. Dis Aquat Org 21:103–108
Magnadottir B (2010) Immunological control of fish diseases. Mar Biotechnol 12:361–379
Manilal A, Sujith S, Kiran GS, Selvin J, Shakir C, Ganghimathi R, Panikkar MVN (2009) Biopotentials of seaweeds collected from southwest coast on India. J Mar Sci Technol 17(1):67–73
Maroux S, Louvard D, Baratti J (1973) The aminopeptidase from hog intestinal brush border. Biochim Biophys Acta 321:282–295
Méthais P, Bieth J (1968) Détermination de l’a-amylase par une microtechnique. Ann Biol Clin 26:133–142
Mommsen TP, Vijayan MM, Moon TW (1999) Cortisol in teleosts: dynamics, mechanisms of action, and metabolic regulation. Rev Fish Biol Fish 9:211–268
Moretti A, Pedini Fernadez-Criado M, Cittolin G, Guidastri R (1999). Manual on hatchery production of sea bass and gilthead sea bream. Volume 1. FAO Fisheries and Aquaculture Department. Rome.
Murray AL, Ponald JP, Alcorn SW, Fairgrieve WT, Shearer KD, Roley D (2003) Effects of various feed supplements containing fish protein hydrolysate or fish processing by products on the innate immune functions of juvenile coho salmon (Oncorhynchus kisutch). Aquaculture 220(1–4):643–653
Parra G, Yufera M (2000) Feeding, physiology and growth responses in first-feeding gilthead seabream (Sparus aurata L.) larvae in relation to prey density. J Exp Mar Bio Ecol 243:1–15
Prol-García MJ, Planas M, Pintado J (2010) Different colonization and residence time of Listonella anguillarum and Vibrio splendidus in the rotifer Brachionus plicatilis determined by real-time PCR and DGGE. Aquaculture 302:26–35
Qi Z, Dierckens K, Defoirdt T, Sorgeloos P, Boon N, Bao Z, Bossier P (2009) Analysis of the evolution of microbial communities associated with different cultures of rotifer strains belonging to different cryptic species of the Brachionus plicatilis species complex. Aquaculture 292:23–29
Ribeiro L, Zambonino-Infate JL, Cahu C, Dinis MT (1999) Development of digestive enzymes in larvae of Solea senegalensis, Kaup, 1858. Aquaculture 179:465–473
Rocha RJ, Ribeiro L, Costa R, Dinis MT (2008) Does the presence of microalgae influence fish larvae prey capture? Aquac Res 39(4):362–369
Rombaut G, Suantika G, Boon N, Maertens S, Dhert P, Top E, Sorgeloos P, Verstraete W (2001) Monitoring of the evolving diversity of the microbial community present in rotifer cultures. Aquaculture 198:237–252
Ronnestad I, Tonheim SK, Fyhn HJ, Rojas-García CR, Kamisaka Y, Koven W, Finn RN, Teriesen BF, Barr Y, Conceição LEC (2003) The supply of amino acids during early feeding stages of marine fish larvae: a review of recent findings. Aquaculture 227:147–164
Rønnestad I, Yúfera M, Ueberschär B, Ribeiro L, Sæle Ø, Boglione C (2013) Feeding behavior and digestive physiology in larval fish: current knowledge, and gaps and bottlenecks in research. Rev Aquacult 5(1):s59–s98
Saurabh S, Sahoo PK (2008) Lysozyme: an important defence molecule of fish innate immune system. Aquac Res 39:223–239
Shields RJ (2001) Larviculture of marine finfish in Europe. Aquaculture 200:55–88
Tandler A, Anav FA, Choshniak I (1995) The effect of salinity on growth rate, survival and swimbladder inflation in gilthead seabream, Sparus aurata, larvae. Aquaculture 135:343–353
Tsalafouta A, Papandroulakis N, Gorissen M, Katharios P, Flik G, Pavlidis M (2014) Ontogenesis of the HPI axis and molecular regulation of the cortisol stress response during early development in Dicentrarchus labrax. Sci Rep 4:552. doi:10.1038/srep05525
Vadstein O, Bergh Ø, Gatesoupe FJ, Galindo-Villegas J, Mulero V, Picchietti S, Scapigliati G, Makridis P, Olsen Y, Dierckens K, Defoirdt T, Boo N, De Schryver P, Bossier P (2013) Microbiology and immunology of fish larvae. Rev Aquacult 5:s1–s25
Verdonck L, Grisez L, Sweetman E, Minkoff G, Sorgeloos P, Ollevier F, Swings J (1997) Vibrios associated with routine productions of Brachionus plicatilis. Aquaculture 149:203–214
Yúfera M, Darias MJ (2007) The onset exogenous feeding in marine fish larvae. Aquaculture 268:53–63
Zambonino-Infante JL, Gisbert E, Sarasquete C, Navarro I, Gutiérrez J, Cahu CL (2008) Ontogeny and physiology of the digestive system of marine fish larvae. In: Cyrino JEP, Bureau D, Kapoor BG (eds) Feeding and digestive functions in fishes. Science Publishers, Enfield, pp 281–348
Acknowledgements
This study was supported by the Portuguese Foundation for Science and Technology. SATA project: PTDC/MAR/112792/2009.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Castanho, S., Califano, G., Soares, F. et al. The effect of live feeds bathed with the red seaweed Asparagopsis armata on the survival, growth and physiology status of Sparus aurata larvae. Fish Physiol Biochem 43, 1043–1054 (2017). https://doi.org/10.1007/s10695-017-0351-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-017-0351-6