Abstract
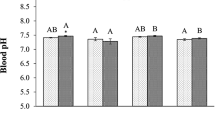
Metals can influence the gonadal steroidogenesis and endocrine systems of fish, thereby affecting their reproduction. The effects of aluminum and manganese in acidic water were investigated on steroidogenesis in sexually mature male Astyanax altiparanae. Whether mature male fish recover from the effects of metals in metal-free water was also assessed. The fish were exposed to 0.5 mg L−1 of isolated or combined aluminum and manganese in acidic pH (5.5) to keep the metals bioavailable. The fish underwent 96 h of acute exposure, and samples were taken 24 and 96 h after the beginning of the experiment. The fish were then maintained in metal-free water for 96 h. Plasma levels of testosterone, 11-ketotestosterone, 17β-estradiol, and cortisol were measured. Acidic water increased the plasma concentration of testosterone and 11-ketotestosterone. Aluminum increased the testosterone levels after 96 h of exposure. Manganese increased the 17β-estradiol levels after 24 h of exposure and maintained at high levels until the end of the experiment. With the exception of acidic pH, which increased cortisol levels after 24 h of exposure, no changes were observed in this corticosteroid during the acute experiment. Aluminum and manganese together also altered steroid levels but without a standard variation. The fish recovered from the effects of most exposure conditions after 96 h in metal-free water. A. altiparanae could use reproductive tactics to trigger changes in testicular steroidogenesis by accelerating spermatogenesis and spermiogenesis, which may interfere with their reproductive dynamics.




Similar content being viewed by others
References
Altli G, Ariyurek SY, Kanakc EG, Canli M (2015) Alterations in the serum biomarkers belonging to different metabolic systems of fish (Oreochromis niloticus) after Cd and Pb exposure. Environ Toxicol Pharm 40:508–515
Arukwe A (2001) Cellular and molecular responses to endocrine-modulators and the impact on fish reproduction. Mar Pollut Bull 42(8):643–655
Camargo MMP, Fernandes MN, Martinez CBR (2009) How aluminium exposure promotes osmoregulatory disturbances in the neotropical freshwater fish Prochilus lineatus. Aquat Toxicol 94:40–46
CETESB—COMPANHIA DE TECNOLOGIA DE SANEAMENTO AMBIENTAL (2012) Relatório de Qualidade das Águas Interiores do Estado de São Paulo. Governo do Estado de São Paulo, Secretaria do Meio Ambiente, São Paulo
CONAMA (2005) Resolução no. 357, de 17 de março de 2005. Ministério do Meio Ambiente. http://www.mma.gov.br/port/conama/res/res05/res35705.pdf. Accessed 20 July 2015
Correia TG, Narcizo AM, Bianchini A, Moreira RG (2010) Aluminum as an endocrine disruptor in female Nile tilapia (Oreochromis niloticus). Comp Biochem Physiol C Toxicol Pharm 151:61–66
Correia TG, Vieira VARO, Moreira RG (2012) Evidências de desregulação endócrina em Astyanax bimaculatus (Linnaeus, 1758) (Characidae). In: XII Congresso Brasileiro de Ecotoxicologia, Porto de Galinhas
Domingues MS, Vicari MR, Abilhoa V, Wamser JP, Cestari MM, Bertollo LAC, Almeida MC, Artoni RF (2007) Cytogenetic and comparative morphology of two allopatric populations of Astyanax altiparanae Garutti and Britski, 2000 (Teleostei: Characidae) from upper rio Paraná basin. Neotrop Ichthyol 5(1):37–44
Garutti V, Britski HA (2000) Descrição de uma espécie nova de Astyanax (Teleostei:Characidae) da bacia do alto Rio Paraná e considerações gerais sobre as demais espécies do gênero na bacia. Comum Mus Ciênc Tecnol PUCRS Série Zoologia 13:65–88
Gensemer RW, Playle RC (1999) The bioavailability and toxicity of aluminum in aquatic environments. Crit Rev Environ Sci Technol 29:315–450
Hampl R, Kubatova J, Starka L (2016) Steroids and endocrine disruptors—history, recent state of art and open questions. J Steroid Biochem Mol Biol 155:217–223
Hontela A, Lacroix A (2006) Heavy metals. In: Norris D, Carr JA (eds) Endocrine disruption: biological bases for health effects in wildlife and humans. Oxford University Press, Nova York, pp 356–374
Hwang UK, Kagawa N, Mugyia Y (2000) Aluminium and cadmium inhibit vitellogenin and its mRNA induction by estradiol-17β in the primary culture of hepatocytes in the rainbow trout (Oncorhynchus mykiss). Gen Comp Endocrinol 119:69–76
Iavicoli I, Fontana L, Bergamaschi A (2009) The effects of metals as endocrine disruptors. J Toxicol Environ Health B Crit Rev 12:206–223
Kime DE, Nash JP (1999) Gamete viability as an indicator of reproductive endocrine disruption in fish. Sci Total Environ 233:123–129
Knapp R, Carlisle SL (2011) Testicular function and hormonal regulation in fishes. In: Norris DO, Lopez KH (eds) Hormones and reproduction of vertebrates: fishes. Elsevier Inc, New York, pp 43–63
Laskey JW, Phelps P (1991) Effect of cadmium and other metal cations on in vivo leydig cell testosterone production. Toxicol Appl Pharm 108:296–306
Lawrence AJ, Arukwe A, Moore M, Sayer M, Thain J (2003) Molecular/cellular processes and the physiological response to pollution. In: Lawrence AJ, Hemingway KL (eds) Effects of pollution on fish: molecular effects and population responses. Blackwell, Oxford, pp 83–133
Lee B, Pine M, Johnson L, Rettori V, Hiney JK, Dees WL (2006) Manganese acts centrally to activate reproductive hormone secretion and pubertal development in male rats. Reprod Toxicol 22:580–585
Lubzens E, Young G, Bobe J, Cerdà J (2010) Oogenesis in teleosts: how fish eggs are formed. Gen Comp Endocrinol 165:367–389
Matthiessen P, Johnson I (2007) Implications of research on endocrine disruption for the environmental risk assessment, regulation and monitoring of chemicals in the European Union. Environ Pollut 146:9–18
Mgbonyebi OP, Smothers CT, Mrotek JJ (1994) Modulation of adrenal cell functions by cadmium salts: 3. Sites affected by CdCl2 during stimulated steroid synthesis. Cell Biol Toxicol 10:35–43
Miura T, Miura C, Ohta T, Nader MR, Todo T, Yamauchi K (1999) Estradiol-17β stimulates the renewal of spermatogonial stem cells in males. Biochem Biophys Res Commun 264:230–234
Monnete MY, McCormick SD (2008) Impacts of short-term acid and aluminum exposure on Atlantic Salmon (Salmo salar) physiology: a direct comparison of parr and smolts. Aquat Toxicol 86:216–226
Narcizo AM, Correia TG, Moreira R (2010) Evaluation of subchronic exposure to aluminum in the reproductive physiology parameters in Astyanax fasciatus. In: Fish Biology Congress, Barcelona, p 177 (abstracts)
Nilsen TO, Ebbenson LOE, Handeland SO, Kroglund F, Finstad B, Angotzi AR, Stefansson SO (2013) Atlantic salmon (Salmo salar L.) smolts require more than two weeks to recover from acid water and aluminum exposure. Aquat Toxicol 142–143:33–44
Norman AW, Litwack G (eds) (1997) Hormones. Academic Press, California
Orsi ML, Carvalho ED, Foresti F (2004) Biologia populacional de Astyanax altiparanae Garutti and Britski (Teleostei, Characidae) do médio Rio Paranapanema, Paraná. Brasil Rev Bras Zool 21(2):207–218
Ozaki Y, Higuchi M, Miura C, Yamaguchi S, Tozawa T, Miura T (2006) Roles of 11β-hydroxysteroid dehydrogenase in fish spermatogenesis. Endocrinology 147:5139–5146
Partridge GJ, Lymbery AJ (2009) Effects of manganese on juvenile mulloway (Argyrosomus japonicus) cultured in water with varying salinity-Implications for inland mariculture. Aquaculture 290:311–316
Pine M, Lee B, Dearth R, Hiney JK, Dees WL (2005) Manganese acts centrally to stimulate luteinizing hormone secretion: a potential influence on female pubertal development. Toxicol Sci 85:880–885
Porto-Foresti F, Castilho-Almeida RB, Senhorini JA, Foresti F (2010) Biologia e criação do lambari-do-rabo-amarelo (Astyanax altiparanae). In: Baldisserotto B, Gomes LC (eds) Espécies Nativas Para Piscicultura No Brasil, vol 1, 2nd edn. Editora UFSM, Santa Maria, pp 101–115
Quintero-Hunter I, Grier HJ, Muscato M (1991) Enhancement of histological detail using yellow as counterstain in period acid Schiff´s hematoxylin staining of glycol methacrylate tissue sections. Biotech Histochem 66:169–172
Sandoy S, Langåker RM (2001) Atlantic salmon and acidification in Southern Norway: a disaster in the 20th century, but a hope for the future? Water Air Soil Pollut 130:1343–1348
Sangalang GB, Freeman HS, Uthe JF, Sperry LS (1990) Effects of diet or liming on steroid hormone metabolism and reproduction in Atlantic Salmon (Salmo salar) held in an acid river. Can J Fish Aquat Sci 47:2422–2430
Schulz RW, França LR, Layere JJ, Legac F, Chiarini-Garcia H, Nobrega RH, Miura T (2010) Spermatogenesis in fish. Gen Comp Endocrinol 165:390–411
Seriani R, Abessa DMS, Moreira LB, Cabrera JPG, Sanches JQ, Silva CLS, Amorim FA, Rivero DHRF, Silva FL, Fitorra LF, Carvalho-Oliveira R, Macchione Mm Ranzani-Paiva MJT (2015) In vitro mucus transportability, cytogenotoxicity, and hematological changes as non-destructive physiological biomarker in fish chronically exposed to metals. Ecotoxicol Environ Saf 112:162–168
Siqueira-Silva DH, Silva APS, Ninhaus-Silveira A, Veríssimo-Silveira R (2015) Morphology of the urogenital papilla and its components ducts in Astyanax altiparanae Garutti and Britski, 2000 (Characiformes: Characidae). Neotrop Ichthyol 13(2):309–316
Teien HC, Salbu B, Kroglund F, Heier LS, Rosseland BO (2007) The influence of colloidal material on aluminium speciation and estimated acid neutralising capacity (ANC). Appl Geochem 22:1202–1208
USEPA (2009) National recommended water quality criteria EPA 4304T. Environmental Protection Agency, Washington
Vieira MC, Torronteras R, Córdoba F, Canalejo A (2012) Acute toxicity of manganese in goldfish Carassius auratus is associated with oxidative stress and organ specific antioxidant responses. Ecotoxicol Environ Saf 78:212–217
Vieira VARO, Correia TG, Moreira RG (2013) Effects of aluminum on the energetic substrates in neotropical freshwater Astyanax bimaculatus (Teleostei: Characidae) females. Comp Biochem Physiol C 157:1–8
Weltzien FA, Taranger GL, Karlsen O, Norberg B (2002) Spermatogenesis and related plasma androgen levels in Atlantic halibut (Hippoglossus hippoglossus L.) Comp. Biochem Physiol A Mol Integr Physiol 132:567–575
Wingfield JC, Sapolsky RM (2003) Reproduction and resist. J Neuroendocrinol 15:711–724
Zelennikov OV, Mosyagina MV, Fedorov KE (1999) Oogenesis inhibition, plasma steroid levels, and morphometric changes in the hypophysis in Russian sturgeon (Acipenser gueldenstaedti Brandt) exposed to low environmental pH. Aquat Toxicol 46:33–42
Acknowledgments
The São Paulo Research Foundation (FAPESP) funded the present study (projects 2008/57687-0 and 2012/50918-1 and scholarship MS 2011/15056-6). The authors thank the fish farm of the Energy Company of São Paulo (CESP) for donating the fish, the LAMEROA staff for their help, and IB/USP for providing the logistics and facilities for the development of this project. All procedures were approved by the Ethics Committee on Animal Use (CEUA), Institute of Biosciences, University of São Paulo under the protocol number 163/2012.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kida, B.M.S., Abdalla, R.P. & Moreira, R.G. Effects of acidic water, aluminum, and manganese on testicular steroidogenesis in Astyanax altiparanae . Fish Physiol Biochem 42, 1347–1356 (2016). https://doi.org/10.1007/s10695-016-0222-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-016-0222-6