Abstract
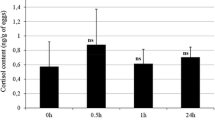
The present study made an attempt to measure the cortisol content, as an indicator of stress response, in rainbow trout embryos which were exposed to different densities and handling stress (air exposure) during incubation. The three densities of experimental embryos at early development stages were considered as 2.55 embryos/cm2 (low density), 5.10 embryos/cm2 (normal density) and 7.65 embryos/cm2 (high density). The cortisol content of eggs (5.09 ± 0.12 ng/g) decreased to 3.68 ± 0.14 ng/g in newly fertilized eggs. Resting level of cortisol dropped at three densities by day 18 of post fertilization. Then, cortisol increased at hatching stage to 1.16 ± 0.11, 1.20 ± 0.12 and 1.21 ± 0.14 ng/g at low, normal and high densities, respectively. There were no statistically significant differences between cortisol concentrations in three densities. The acute handling stress test (5-min out-of-water), conducted on embryos (48 h post fertilization, organogenesis and eyed stage) in three densities, revealed no differences in whole-body cortisol levels between stressed and unstressed experimental groups. At hatching stage in low-density group, level of cortisol increased but the difference with the pre-stress levels was not statistically significant. Furthermore, significant differences in cortisol levels of stressed and unstressed embryos were detected on hatching in normal and high density groups [1.20 ± 0.12 at time 0–1.49 ± 0.11 ng/g at 1 hps (hours post stress) and from 1.21 ± 0.14 at time 0 to 1.53 ± 0.10 ng/g at 3 hps, respectively]. The results showed no difference in profile of cortisol in different densities, but acute stress conducted on embryos, incubated in different densities, revealed differences in cortisol stress response at hatching between normal and high density, which lead to cortisol increase at hatching time. It indicates that the lag time in the cortisol response to stressors immediately after hatching does not occur when the siblings were stressed during the embryo stage. Results, finally, indicated that hypothalamus–pituitary–interrenal axis was active and responded to an acute stressor under normal and high density, but it is unresponsive to a stressor around hatching under low density.





Similar content being viewed by others

References
Applebaum SL, Wilson CA, Holt GJ, Nunez BS (2010) The onset of cortisol synthesis and the stress response is independent of changes in CYP11B or CYP21 mRNA levels in larval red drum (Sciaenops ocellatus). Gen Comp Endocrinol 165:269–276
Auperin B, Geslin M (2008) Plasma cortisol response to stress in juvenile rainbow trout is influenced by their life history during early development and by egg cortisol content. Gen Comp Endocrinol 158:234–239
Barry TP, Ochiai M, Malison JA (1995a) In vitro effects of ACTH on interrenal corticosteroidogenesis during early larval development in rainbow trout. Gen Comp Endocrinol 99:382–387
Barry TP, Malison JA, Held JA, Parrish JJ (1995b) Ontogeny of the cortisol stress response in larval rainbow trout. Gen Comp Endocrinol 97:57–65
Barton BA (2002) Stress in fishes: a diversity of responses with particular reference to changes in circulating corticosteroids. Integr Comp Biol 42:517–525
Barton BA, Iwama GK (1991) Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annu Rev Fish Dis 1:3–26
Bernier NJ, Peter RE (2001) The hypothalamic–pituitary–interrenal axis and the control of food intake in teleost fish. Comp Biochem Physiol 129B:639–644
Bromage NR, Jones J, Randall C, Thrush M, Davies B, Springate J, Duston J, Barker G (1992) Broodstock management, fecundity, egg quality and the timing of egg production in the rainbow trout (Oncorhynchus mykiss). Aquaculture 100:141–166
De Jesus EG, Hirano T (1992) Changes in whole body concentrations of cortisol, thyroid hormones, and sex steroids during early development of the chum salmon, Oncorhynchus keta. Gen Comp Endocrinol 85:55–61
De Jesus EG, Hirano T, Inui Y (1991) Changes in cortisol and thyroid hormone concentrations during early development and metamorphosis in the Japanese flounder, Paralichthys olivaceus. Gen Comp Endocrinol 82:369–376
Deane EE, Woo NYS (2003) Ontogeny of thyroid hormones, cortisol, hsp70 and hsp90 during silver sea bream larval development. Life Sci 72:805–818
Falahatkar B, Akhavan SR, Efatpanah I, Meknatkhah B (2012) Primary and secondary responses of a teleostean, pikeperch Sander lucioperca, and a chondrostean, Persian sturgeon Acipenser persicus juveniles, to handling during transport. N Am J Aquac 74:241–250
Falahatkar B, Akhavan SR, Ghaedi GR (2013) Egg cortisol response to stress at early stages of development in Persian sturgeon Acipencer persicus. Aquac Int 21:215–223
Feist G, Schreck CB (2002) Ontogeny of the stress response in chinook salmon, Oncorhynchus tshawytscha. Fish Physiol Biochem 25:31–40
Hwang PP, Wu SM (1993) Role of cortisol in hypo-osmoregulation in larvae of tilapia (Oreochromis mossambicus). Gen Comp Endocrinol 92:318–324
Hwang PP, Wu SM, Lin JH, Wu LS (1992) Cortisol content of eggs and larvae of teleosts. Gen Comp Endocrinol 86:189–196
Iwama GK, Afonso LOB, Vijayan MM (2005) Stress in fish. In: Evans DH, Claiborne JB (eds) The physiology of fishes, 3rd edn. CRC Press, Boca Raton, FL, pp 319–342
Jentoft S, Held JA, Malison JA, Barry TP (2002) Ontogeny of the cortisol stress response in yellow perch (Perca flavescens). Fish Physiol Biochem 26:371–378
Jentoft S, Aastveit AH, Torjesen PA, Andersen Ø (2005) Effects of stress on growth, cortisol and glucose levels in non-domesticated Eurasian perch (Perca fluviatilis) and domesticated rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol 141A:353–358
Kiilerich P, Kristiansen K, Madsen SS (2007) Cortisol regulation of ion transporter mRNA in Atlantic salmon gill and the effect of salinity on the signaling pathway. J Endocrinol 194:417–427
Kime DE, Nash JP (1999) Gamete viability as an indicator of reproductive endocrine disruption in fish. Sci Total Environ 233:123–129
McCormick SD (2001) Endocrine control of osmoregulation in teleost fish. Am Zool 41:781–794
McGeer JC, Baranyi L, Iwama GK (1991) Physiological responses to challenge tests in six stocks of coho salmon (Oncorhynchus kisutch). Can J Fish Aquat Sci 48:1761–1771
Mileva VR, Gilmour KM, Balshine S (2011) Effects of maternal stress on egg characteristics in a cooperatively breeding fish. Comp Biochem Physiol 158A:22–29
Milla S, Wang N, Mandiki SNM, Kestemont P (2009) Corticosteroids: friends or foes of teleost fish reproduction? Comp Biochem Physiol 153A:242–251
Mommsen TP, Vijayan MM, Moon TW (1999) Cortisol in teleosts: dynamics, mechanisms of action, and metabolic regulation. Rev Fish Biol Fish 9:211–268
Øverli Ø, Sørensen C, Kiessling A, Pottinger TG, Gjøen HM (2006) Selection for improved stress tolerance in rainbow trout (Oncorhynchus mykiss) leads to reduced feed waste. Aquaculture 261:776–781
Pankhurst NW, Van Der Kraak G (1997) Effects of stress on reproduction and growth of fish. In: Iwama GK, Pickering AD, Sumpter JP, Schreck CB (eds) Fish stress and health in aquaculture. Cambridge University Press, Cambridge, pp 73–93
Pottinger TG, Mosuwe E (1994) The corticosteroidogenic response of brown and rainbow trout alevins and fry to environmental stress during a ‘critical period’. Gen Comp Endocrinol 95:350–362
Pottinger AD, Pickering TG, Hurley MA (1992) Consistency in the stress response of individuals of two strains of rainbow trout, Oncorhynchus mykiss. Aquaculture 103:275–289
Pottinger TG, Moran TA, Morgan JAW (1994) Primary and secondary indexes of stress in the progeny of rainbow trout (Oncorhynchus mykiss) selected for high and low responsiveness to stress. J Fish Biol 44:149–163
Pourhosein Sarameh S, Falahatkar B, Azari Takami G, Efatpanah I (2012) Effects of different photoperiods and handling stress on spawning and reproductive performance of pikeperch Sander lucioperca. Anim Reprod Sci 132:213–222
Poursaeid S, Falahatkar B, Mojazi Amiri B, Van Der Kraak G (2012) Effects of long-term cortisol treatments on gonadal development, sex steroids levels and ovarian cortisol content in cultured great sturgeon Huso huso. Comp Biochem Physiol 163A:111–119
Raine JC, Leatherland JF (2003) Trafficking of L-triiodothyronine between ovarian fluid and oocytes of rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol 136B:267–274
Ramsay JM, Feist GW, Varga ZM, Westerfield MM, Kent L, Schreck CB (2006) Whole-body cortisol is an indicator of crowding stress in adult zebrafish, Danio rerio. Aquaculture 258:565–574
Sampath-Kumar R, Byers RE, Munro AD, Lam TJ (1995) Profile of cortisol during ontogeny of the Asian seabass, Lates calcarifer. Aquaculture 132:349–359
Sampath-Kumar R, Lee ST, Tan CH, Munro AD, Lam TJ (1997) Biosynthesis in vivo and excretion of cortisol by fish larvae. J Exp Zool 277:337–344
Schreck CB, Contreras-Sanchez W, Fitzpatrick MS (2001) Effects of stress on fish reproduction, gamete quality, and progeny. Aquaculture 197:3–24
Simontacchi C, Negrato E, Pazzaglia M, Bertotto D, Poltronieri C, Radaelli G (2008) Whole-body concentrations of cortisol and sex steroids in white sturgeon (Acipenser transmontanus, Richardson 1836) during early development and stress response. Aquacult Int 17:7–14
Stouthart AJHX, Lucassen ECHET, van Strien FJC, Balm PHM, Lock RAC, Wendelaar Bonga SE (1998) Stress responsiveness of the pituitary-interrenal axis during early life stages of common carp (Cyprinus carpio). J Endocrinol 157:127–137
Stratholt ML, Donaldson EM, Liley NR (1997) Stress induced elevation of plasma cortisol in adult female coho salmon (Oncorhynchus kisutch), is reflected in egg cortisol content, but does not appear to affect early development. Aquaculture 158:141–153
Szisch V, Papandroulakis N, Fanouraki E, Pavlidis M (2005) Ontogeny of the thyroid hormones and cortisol in the gilthead sea bream, Sparus aurata. Gen Comp Endocrinol 142:186–192
Tagawa M, Tanaka M, Matsumoto S, Hirano T (1990) Thyroid hormones in eggs of various freshwater, marine and diadromous teleosts and their changes during egg development. Fish Physiol Biochem 8:515–520
Wendelaar Bonga SE (1997) The stress response in fish. Physiol Rev 77:591–625
Acknowledgments
This work was financially supported by a research grant of Khorramshahr University of Marine Science and Technology. We thank all the members of the Shahid Motahari Coldwater Fish Genetic and Breeding Research Center, Yasouj, Iran for supplying the experimental eggs and rearing facilities and also the microbiology laboratory, Faculty of Veterinary Medicine, University of Tehran for kind assistance in the cortisol measurement. Also special thank to G. Van Der Kraak for some comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghaedi, G., Falahatkar, B., Yavari, V. et al. The onset of stress response in rainbow trout Oncorhynchus mykiss embryos subjected to density and handling. Fish Physiol Biochem 41, 485–493 (2015). https://doi.org/10.1007/s10695-014-9999-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-014-9999-3