Abstract
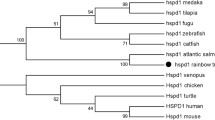
The glucose-regulated protein, 94 kDa (GRP94), is an endoplasmic reticulum (ER)-localized heat shock protein that plays among other functions a crucial role in folding and exports of Toll-like receptors (TLRs) and some other immune-relevant factors. We identified two copies of the GRP94-encoding gene in rainbow trout sharing 91 % DNA sequence identity. The conceptually translated ORFs encode a 795-aa GRP94a and a 510-aa GRP94b protein variant, respectively, with characteristic domains and amino acid residues. However, the shorter variant lacks motifs required for its localization in the ER and might thus represent an isoform of the putative mammalian ortholog GRP94a. Heat stress only slightly affects the expression of the two GRP94-encoding trout genes, as reported for mammals. We recorded the abundances of transcripts coding for both GRP94 variants as well as for a broad panel of TLRs representing their potential targets. In embryonic and larval trout, only the mRNAs encoding TLR1, −2, −9, and −20 were found in significant concentrations, while the expression of nine other TLRs was hardly detectable. The GRP94a-encoding gene showed constantly high expression levels indicating that this isoform is vitally required throughout the life cycle of rainbow trout. The concentration of the GRP94b-encoding mRNA was only ~0.1 % compared to the GRP94a mRNA level. These structural and gene expression data together suggest that the two GRP94 gene products fulfill different physiological roles.



Similar content being viewed by others
Abbreviations
- Aa:
-
Amino acid
- BLAST:
-
Basic local alignment search tool
- DAMP:
-
Damage-associated molecular patterns
- dpf:
-
Days post-fertilization
- ER:
-
Endoplasmic reticulum
- GRP94:
-
Glucose-regulated protein 94
- HATPase:
-
Histidine kinase-like adenine nucleotide binding
- HSP:
-
Heat shock proteins
- HSP90B1:
-
Heat shock protein 90 kDa beta, member 1
- PAMP:
-
Pathogen-associated molecular patterns
- TLR:
-
Toll-like receptors
References
Allendorf F, Thorgaard G (1984) Tetraploidy and the evolution of salmonid fishes. In: Turner B (ed) Evolutionary genetics of fishes. Springer, US, pp 1–53
Bailey GS, Poulter RTM, Stockwell PA (1978) Gene duplication in tetraploid fish: model for gene silencing at unlinked duplicated loci. Proc Natl Acad Sci USA 75:5575–5579
Bendtsen JD, Nielsen H, von Heijne G, Brunak S (2004) Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 340:783–795
Biswas C, Ostrovsky O, Makarewich CA, Wanderling S, Gidalevitz T, Argon Y (2007) The peptide-binding activity of GRP94 is regulated by calcium. Biochem J 405:233–241
Bowers RM, Lapatra SE, Dhar AK (2008) Detection and quantitation of infectious pancreatic necrosis virus by real-time reverse transcriptase-polymerase chain reaction using lethal and non-lethal tissue sampling. J Virol Methods 147:226–234
Bustin SA (2000) Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol 25:169–193
Chen B, Piel WH, Gui L, Bruford E, Monteiro A (2005) The HSP90 family of genes in the human genome: insights into their divergence and evolution. Genomics 86:627–637
Combet C, Blanchet C, Geourjon C, Deleage G (2000) NPS@: network protein sequence analysis. Trends Biochem Sci 25:147–150
Drummond IA, Lee AS, Resendez E Jr, Steinhardt RA (1987) Depletion of intracellular calcium stores by calcium ionophore A23187 induces the genes for glucose-regulated proteins in hamster fibroblasts. J Biol Chem 262:12801–12805
Eletto D, Dersh D, Argon Y (2010) GRP94 in ER quality control and stress responses. Semin Cell Dev Biol 21:479–485
Force A, Lynch M, Pickett FB, Amores A, Yan YL, Postlethwait J (1999) Preservation of duplicate genes by complementary, degenerative mutations. Genetics 151:1531–1545
Gupta RS (1995) Phylogenetic analysis of the 90 kD heat shock family of protein sequences and an examination of the relationship among animals, plants, and fungi species. Mol Biol Evol 12:1063–1073
Heinecke RD, Chettri JK, Buchmann K (2014) Adaptive and innate immune molecules in developing rainbow trout, Oncorhynchus mykiss eggs and larvae: expression of genes and occurrence of effector molecules. Fish Shellfish Immunol 38:25–33
Jault C, Pichon L, Chluba J (2004) Toll-like receptor gene family and TIR-domain adapters in Danio rerio. Mol Immunol 40:759–771
Kim KS, Kim YK, Lee AS (1990) Expression of the glucose-regulated proteins (Grp94 and Grp78) in differentiated and undifferentiated mouse embryonic-cells and the use of the Grp78 promoter as an expression system in embryonic-cells. Differentiation 42:153–159
Kumar S, Tamura K, Nei M (2004) MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform 5:150–163
Letunic I, Doerks T, Bork P (2009) SMART 6: recent updates and new developments. Nucleic Acids Res 37:D229–D232
Liu B, Li Z (2008) Endoplasmic reticulum HSP90b1 (gp96, grp94) optimizes B-cell function via chaperoning integrin and TLR but not immunoglobulin. Blood 112:1223–1230
Liu B, Yang Y, Qiu Z, Staron M, Hong F, Li Y, Wu S, Li Y, Hao B, Bona R, Han D, Li Z (2010) Folding of Toll-like receptors by the HSP90 paralogue gp96 requires a substrate-specific cochaperone. Nat Commun 1:79
Marzec M, Eletto D, Argon Y (2012) GRP94: an HSP90-like protein specialized for protein folding and quality control in the endoplasmic reticulum. Biochim Biophys Acta 1823:774–787
Meunier L, Usherwood YK, Chung KT, Hendershot LM (2002) A subset of chaperones and folding enzymes form multiprotein complexes in endoplasmic reticulum to bind nascent proteins. Mol Biol Cell 13:4456–4469
Obermann WM, Sondermann H, Russo AA, Pavletich NP, Hartl FU (1998) In vivo function of Hsp90 is dependent on ATP binding and ATP hydrolysis. J Cell Biol 143:901–910
Ostrovsky O, Makarewich CA, Snapp EL, Argon Y (2009) An essential role for ATP binding and hydrolysis in the chaperone activity of GRP94 in cells. Proc Natl Acad Sci USA 06:11600–11605
Phelan PE, Mellon MT, Kim CH (2005) Functional characterization of full-length TLR3, IRAK-4, and TRAF6 in zebrafish (Danio rerio). Mol Immunol 42:1057–1071
Pietretti D, Scheer MH, Wiegertjes GF (2011) Molecular and functional characterization TLR20: possible role in recognition of protozoan parasites. Book of Abstracts of the 15th international conference on disease of fish and shellfish: 252
Randow F, Seed B (2001) Endoplasmic reticulum chaperone gp96 is required for innate immunity but not cell viability. Nat Cell Biol 3:891–896
Rebl A, Goldammer T, Seyfert HM (2010) Toll-like receptor signaling in bony fish. Vet Immunol Immunopathol 134:139–150
Rebl A, Köbis JM, Fischer U, Takizawa F, Verleih M, Wimmers K, Goldammer T (2011) MARCH5 gene is duplicated in rainbow trout, but only fish-specific gene copy is up-regulated after VHSV infection. Fish Shellfish Immunol 6:1041–1050
Rebl A, Verleih M, Köbis J, Kühn C, Wimmers K, Köllner B, Goldammer T (2013) Transcriptome profiling of gill tissue in regionally bred and globally farmed rainbow trout strains reveals different strategies for coping with thermal stress. Mar Biotechnol 15:445–460
Robert J, Cohen N (1998) Evolution of immune surveillance and tumor immunity: studies in Xenopus. Immunol Rev 166:231–243
Roberts RJ, Agius C, Saliba C, Bossier P, Sung YY (2010) Heat shock proteins (chaperones) in fish and shellfish and their potential role in relation to fish health: a review. J Fish Dis 33:789–801
Shiu RPC, Pouyssegur J, Pastan I (1977) Glucose depletion accounts for induction of 2 transformation-sensitive membrane proteins in rous-sarcoma virus-transformed chick-embryo fibroblasts—(glucose starvation membrane proteins). Proc Natl Acad Sci USA 74:3840–3844
Soldano KL, Jivan A, Nicchitta CV, Gewirth DT (2003) Structure of the N-terminal domain of GRP94: basis for ligand specificity and regulation. J Biol Chem 278:48330–48338
Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT (2012) PAMPs and DAMPs: signal 0 s that spur autophagy and immunity. Immunol Rev 249:158–175
van der Sar AM, Stockhammer OW, van der Laan C, Spaink HP, Bitter W, Meijer AH (2006) MyD88 innate immune function in a zebrafish embryo infection model. Infect Immun 74:2436–2441
Wilkins MR, Gasteiger E, Bairoch A, Sanchez JC, Williams KL, Appel RD, Hochstrasser DF (1999) Protein identification and analysis tools in the ExPASy server. Methods Mol Biol 112:531–552
Yang Y, Liu B, Dai J, Srivastava PK, Zammit DJ, Lefrancois L, Li Z (2007) Heat shock protein gp96 is a master chaperone for toll-like receptors and is important in the innate function of macrophages. Immunity 26:215–226
Acknowledgments
We would like to thank the LFA-MV Institute for Fisheries (Born and Hohen Wangelin, Germany) and the fish farm BIMES (Frauenmark, Germany) for breeding and providing the fish sample material. We are grateful to B. Schöpel, I. Hennings, and M. Fuchs for dedicated technical assistance. This work benefited equally from funding by the DFG-Grant SE 326/16-1 from the German Research Foundation and by Grant No. AU11040 from the European Social Fund (ESF) and Ministry of Education, Science and Culture of Mecklenburg-Western Pomerania.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10695_2014_9979_MOESM1_ESM.tif
Amino acid sequence alignment of GRP94a (upper row) and GRP94b (lower row) proteins from rainbow trout Oncorhynchus mykiss (OM) with the ortholog sequences of pufferfishes Takifugu rubripes (TR), Tetraodon nigroviridis (TN), Japanese flounder Paralichthys olivaceus (PO), zebrafish Danio rerio (DR), and grass carp Ctenopharyngodon idella (CI) (for accession numbers see Fig. 1). Identical aa residues are labeled by black underlay. Characteristic domains are indicated with gray underlay above the alignment. 1 (TIFF 5067 kb)
Rights and permissions
About this article
Cite this article
Rebl, A., Brietzke, A., Goldammer, T. et al. GRP94 is encoded by two differentially expressed genes during development of rainbow trout (Oncorhynchus mykiss). Fish Physiol Biochem 40, 1917–1926 (2014). https://doi.org/10.1007/s10695-014-9979-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-014-9979-7