Abstract
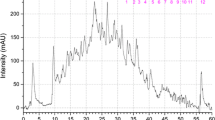
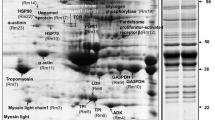
In this study, the proteome profile of European sea bass (Dicentrarchus labrax) muscle was analyzed using two-dimensional electrophoresis (2-DE) and tandem mass spectrometry with the aim of providing a more detailed characterization of its specific protein expression profile. A highly populated and well-resolved 2-DE map of the sea bass muscle tissue was generated, and the corresponding protein identity was provided for a total of 49 abundant protein spots. Upon Ingenuity Pathway Analysis, the proteins mapped in the sea bass muscle profile were mostly related to glycolysis and to the muscle myofibril structure, together with other biological activities crucial to fish muscle metabolism and contraction, and therefore to fish locomotor performance. The data presented in this work provide important and novel information on the sea bass muscle tissue-specific protein expression, which can be useful for future studies aimed to improve seafood traceability, food safety/risk management and authentication analysis. This work is also important for understanding the proteome map of the sea bass toward establishing the animal as a potential model for muscular studies.



Similar content being viewed by others
References
Addis MF, Cappuccinelli R, Tedde V, Pagnozzi D, Porcu MC, Bonaglini E, Roggio T, Uzzau S (2010) Proteomic analysis of muscle tissue from gilthead sea bream (sparus aurata, l.) farmed in offshore floating cages. Aquaculture 309:245–252
Addis MF, Pisanu S, Preziosa E, Pagnozzi D, Bernardini G, Roggio T, Uzzau S, Saroglia M, Terova G (2012) 2D DIGE/MS to investigate the impact of slaughtering techniques on postmortem integrity of fish filet proteins. J Proteomics 75:3654–3664
Chevallet M, Luche S, Rabilloud T (2006) Silver staining of proteins in polyacrylamide gels. Nat Protoc 1:1852–1858
Civera T (2003) Species identification and safety of fish products. Vet Res Commun 27:481–489
Fornè I, Abiàn J, Cerdà J (2010) Fish proteome analysis: model organism and non-sequenced species. Proteomics 10:858–872
Martinez I, Friis TJ (2004) Application of proteome analysis to seafood authentication. Proteomics 4:347–354
Perez J, Garcia-Vazquez E (2004) Genetic identification of nine hake species for detection of commercial fraud. J Food Prot 67:2792–2796
Perez J, Alvarez P, Martinez JL (2005) Genetic identification of hake and megrim eggs in formaldehyde-fixed plankton samples. ICES J Mar Sci 62:908–914
Piñeiro C, Barros-Velázquez J, Sotelo CG, Gallardo JM (1999) The use of two-dimensional electrophoresis in the characterization of the water-soluble protein fraction of commercial flat fish species. Z Lebensm Unters Forsch 208:342–348
Reddish JM, St-Pierre N, Nichols A, Green-Church K, Wick M (2008) Proteomic analysis of proteins associated with body mass and length in yellow perch, Perca flavescens. Proteomics 8:2333–2343
Schiavone R, Zilli L, Storelli C, Vilella S (2008) Identification by proteome analysis of muscle proteins in sea bream (Sparus aurata). Eur Food Res Technol 227:1403–1410
Terova G, Addis MF, Preziosa E, Pisanu S, Pagnozzi D, Biosa G, Gornati R, Bernardini G, Roggio T, Saroglia M (2011) Effect of post mortem storage temperature on sea bass (Dicentrarchus labrax) muscle protein degradation: analysis by 2-D DIGE and mass spectrometry. Proteomics 11:2901–2910
The UniProt Consortium The universal protein resource (UniProt) (2008) Nucleic Acids Res 36, D190-D195
Toffoli D, Hrbek T, de Araujo MLG, De Almeida MP (2008) A test of the utility of DNA Barcoding in the radiation of the freshwater stingray genus Potamotrygon (Potamotrygonidae, Myliobatiformes). Genet Mol Biol 31:324–336
Zhou X, Ding Y, Wang Y (2012) Proteomic: present and future in fish, shellfish and seafood. Rev Aquac 4:11–20
Acknowledgments
This work has been funded under the ARRAINA project N°288925: Advanced Research Initiatives for Nutrition and Aquaculture. The views expressed in this work are the sole responsibility of the authors and do not necessary reflect the views of the European Commission.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Terova, G., Pisanu, S., Roggio, T. et al. Proteomic profiling of sea bass muscle by two-dimensional gel electrophoresis and tandem mass spectrometry. Fish Physiol Biochem 40, 311–322 (2014). https://doi.org/10.1007/s10695-013-9855-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-013-9855-x