Abstract
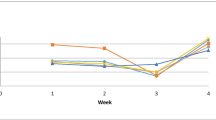
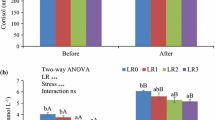
Two bacterial strains, Lactobacillus fructivorans, isolated from sea bream (Sparus aurata) gut, and Lactobacillus plantarum, isolated from human faeces, were administered simultaneously, during sea bream development, using Brachionus plicatilis and/or Artemia salina as vectors. The probiotic treatment significantly affected gut colonization. To test the probiotic influence on stress responsiveness, sea bream fry, 47 days post-hatching (p.h.), were subjected to pH stress (from 8.6 to 6.3) and cumulative mortality, cortisol levels and HSP70 gene expression were analysed. Cortisol was selected, since under stress conditions its level increases. HSP70 was selected with consideration of its wide involvement in response to a great number of injuries, and because it protects cells probably by binding and refolding damaged proteins. The results obtained indicated that the administration of probiotic to sea bream fry induced higher HSP70 levels, indicating a greater potentiality to respond to the harmful conditions possibly present in fish farms. This hypothesis is supported by the fact that the levels of cortisol found were significantly lower (P < 0.05) in both groups under probiotic treatment. When pH was used as a stressor, it induced a higher cumulative mortality in the control; the mortality was found to be significantly lower in both treated groups. Interestingly, a significant increase (P < 0.05) in HSP70 gene expression was observed in all stressed groups. These results suggest an improvement in tolerance to acute stress of fry fed with probiotics.
Similar content being viewed by others
References
Barton BA, Iwama GK (1991) Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annu Rev Fish Dis 1:3–26
Barton BA, Schreck CB, Barton LD (1987) Effects of chronic cortisol administration and daily acute stress on growth, physiological conditions, and stress responses in juvenile rainbow trout. Dis Aquat Org 2:173–185
Basu N, Nakano T, Grau EG, Iwama GK (2001) The effects of cortisol on heat shock protein 70 levels in two fish species. Gen Comp Endocrinol 124:97–105
Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR (2000) Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9␣to the Apaf-1 apoptosome. Nat Cell Biol 2:469–475
Bernet MF, Brassart D, Neeser JR, Servin AL (1994) Lactobacillus acidophilus LA 1 binds to cultured human intestinal cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut 35(4):483–489
Bernet-Camard MF, Lievin V, Brassart D, Neeser JR, Servin AL, Hudault S (1997) The human Lactobacillus acidophilus strain LA1 secretes a nonbacteriocin antibacterial substance(s) active in vitro and in vivo. Appl Environ Microbiol 63(7):2747–2753
Boone AN, Vijayan MM (2002) Glucocorticoid-mediated attenuation of the HSP70 response in trout hepatocytes involves the proteasome. Am J Regul Integr Comp Physiol 283:680–687
Brownell CL (1980) Water quality requirements for first-feeding in marine fish larvae. II. pH, oxygen and carbon dioxide. J Exp Mar Biol Ecol 44:269–283
Carnevali O, Maradonna F (2003) Exposure to xenobiotic compounds: looking for new biomarkers. Gen Comp Endocrinol 131:203–209
Carnevali O, Zamponi MC, Sulpizio R, Rollo A, Nardi M, Orpianesi C, Silvi S, Caggiano M, Polzonetti AM, Cresci A (2004) Administration of probiotic strain to improve sea bream wellness during development. Int Aquacul 12:377–386
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem 162(1):156–159
Coconnier MH, Bernet MF, Kerneis S, Chauviere G, Fourniat J, Servin AL (1993) Inhibition of adhesion of enteroinvasive pathogens to human intestinal Caco-2 cells by Lactobacillus acidophilus strain LB decreases bacterial invasion. FEMS Microbiol Lett 110(3):299–305
Daw MA, Falkiner FR (1996) Bacteriocins: nature, function and structure. Micron 27(6):467–479
Ellsaesser CF, Clem LW (1986a) Haematological and immunological changes in channel catfish stressed by handling and transport. J Fish Biol 28:511–521
Ellsaesser CF, Clem LW (1986b) Haematological and immunological changes in channel catfish stressed by␣handling and transport. Dev Comp Immunol 10:149
Finlay BB, Falkow S (1990) Salmonella interactions with polarized human intestinal Caco-2 epithelial cells. J Infect Dis 162(5):1096–1106
Garrido C, Girbuxani S, Ravagnan L, Kroemer G (2001) Heat shock proteins: endogenous modulators of apoptotic cell death. Biochem Biophys Res Commun 286:433–442
Gatesoupe FJ (1999) The use of probiotics in aquaculture. Aquaculture 180:147–165
Gomez-Gill B, Roque A, Turnbull JF (2000) The use and selection of probiotic bacteria for use in the culture of larval aquatic organisms. Aquaculture 191:259–270
Guillard RRL (1975) Culture of phytoplankton for feeding marine invertebrates. In: Smith WL, Chanley MH (eds) Culture of marine invertebrate animals. Plenum, New York, pp 26–60
Havenaar R, Ten Brink B, Huis in’t Veld JHJ (1992) Selection of strains for probiotics use. In: Fuller R (ed) Probiotics, the scientific basis. Chapman and Hall, London, pp 209–224
Holdeman VL, Cato EP, Moore CWE (1997) Anaerobe laboratory manual, 4th edn. Virginia Polytechnic Institute and State University, Blacksburg, VA
Kamler E (1992) Early life history of fish: an energetics approach. In: Fish and Fisheries, Series 4. Chapman and Hall, London, p 267
Muroga K, Higashi M, Keitoku H (1987) The isolation of intestinal microflora of farmed red sea bream (Acanthopagrus schlegeli) at larval and juvenile stages. Aquaculture 65:79–88
Nakano K, Iwama G (2002) The 70-kDa heat shock protein response in two intertidal sculpins, Oligocottus maculosus and O. snyderi: relationship of HSP70 and thermal tolerance. Comp Biochem Physiol A Mol Integr Physiol 133(1):79–94
Norris CE, Di Iorio PJ, Schultz RJ, Hightower LE (1995) Variation in heat shock proteins within tropical and desert species of poeciliid fishes. Mol Biol Evol 12(6):1048–1062
Nousiainen JG, Setälä J (1993) Lactic acid bacteria as animal probiotics. In: Salminem S, von Wright A (eds)␣Lactic acid bacteria. Dekker, New York, pp 315–356
Øie G, Makridis P, Reitan KL, Olsen Y (1997) Protein and carbon utilization of rotifers (Brachionus plicatilis) in first feeding of turbot larvae (Scophtalamus maximus L.). Aquaculture 153:103–122
Olsen Y, Reitan KI, Vadstein O (1993) Dependence of temperature on loss rate of rotifers, lipids and fatty acids in starved Brachionus plicatilis cultures. Hydrobiology 255/256:13–20
Ravagnan L, Gurbuxani S, Susin S, Masse C, Daugas E, Zampami N, Mak T, Jaattela M, Penninger J, Garrido C, Kroemer G (2001) Heat shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol 3(9):839–843
Reitan KI, Rainuzzo JR, Øie G, Olsen Y (1993) Nutritional effects of algal addition in first feeding of turbot (Scophtalamus maximus L.) larvae. Aquaculture 118:257–275
Ringø E (1999) Lactic acid bacteria in fish: antibacterial effect against fish pathogens. In: Krogdahl Å, Mathiesen SD, Pryme I (eds) Effects of antinutrients on the nutritional value of legume diets, vol 8. EEC, Luxembourg, pp 70–75
Ringø E, Gatesoupe FJ (1998) Lactic acid bacteria in fish: a review. Aquaculture 160:177–203
Sakata T (1990) Microflora in the digestive tract of fish and shell-fish. In: Lésel R (ed) Microbiology in poecilotherms. Elsevier, Amsterdam, pp 171–176
Saleh A, Srinivasula SM, Balkir L, Robbins PD, Alnemir ES (2000) Negative regulation of the Apaf-1 apoptosome by HSP70. Nat Cell Biol 2:476–483
Salminen S, Bouley C, Boutron-Ruault MC, Cummings JH, Franck A, Gibson GR, Isolauri E, Moreau MC, Roberfroid M, Rowland I (1998) Functional food science and gastrointestinal physiology and function. J Nutr 80:S147–S171
Schaedler RW, Dubos RJ (1962) The fecal flora of various strains of mice. Its bearing on their susceptibility to endotoxin. J Exp Med 115:1149–1160
Schreck CB (1981) Stress and compensation in teleostean fishes: response to social and physical factors. In: Pickering AD (ed) Stress and fish. Academic Press, London, pp 295–321
Silvi S, Verdenelli MC, Orpianesi C, Cresci A (2003) EU project Crownalife: functional foods, gut microflora and healthy ageing. Isolation and identification of Lactobacillus and Bifidobacterium strains from faecal samples of elderly subjects for a possible probiotic use in functional foods. J Food Eng 56/2–3:195–200
Strøm E, Ringø E (1993) Changes in bacterial flora in developing cod, Gadus morhua (L.), larvae after inoculation of Lactobacillus plantarum in the water. In: Walther B, Fyhn HJ (eds) Physiological and biochemical aspects of fish larval development. University of Bergen, Norway, pp 226–228
Tripp RA, Maule AG, Schreck CB, Kaattari SL (1987) Cortisol mediated suppression of salmonid lymphocyte responses in vitro. Dev Comp Immunol 11:565–576
Van Weerd JH, Komen J (1998) The effects of chronic stress on growth in fish: a critical appraisal. Comp Biochem Physiol Part A 120:107–112
Westerdahl A, Olsson JC, Kjelleberg S, Conway PL (1991) Isolation and characterization of turbot (Scophtalmus maximus) associated bacteria with inhibitory effects against Vibrio anguillarum. Appl Environ Microbiol 57:2223–2228
Westernhagen H (1988) Sublethal effects of pollutants on fish eggs and larvae. In: Hoar WS, Randall DJ (eds) Fish physiology, vol XI, Part A. Academic Press, San Diego
Young RA (1990) Stress proteins and immunology. Ann Rev Immunol 8:401–420
Acknowledgements
The authors wish to thank Agata Intini, Dr. Rino Grilli, Dr. Belaid Amalou, Stefano e Gianfranco Lo Parco, Dr. Fabrizio Basile, and Roberto Lo Martire (Panittica Pugliese) for helping in fish handling.
This study was supported by the “Fondi di Ateneo” grant awarded to O. Carnevali.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rollo, A., Sulpizio, R., Nardi, M. et al. Live microbial feed supplement in aquaculture for improvement of stress tolerance. Fish Physiol Biochem 32, 167–177 (2006). https://doi.org/10.1007/s10695-006-0009-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-006-0009-2