Abstract
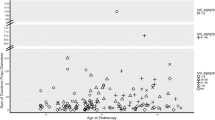
Attenuated polyposis could be defined as a variant of familial adenomatous polyposis (FAP) in which synchronous polyps of the large bowel range between 10 and 99. We analysed all cases of attenuated polyposis observed over the last 30 years with the objectives: (A) to classify the disease according to different type and proportion of polyps; (B) To ascertain the contribution of APC and MutYH genes; (C) to discover features which could arise the suspicion of mutations; (D) To obtain indications for management and follow-up. 84 individuals in 82 families were studied. Polyps were classified into four groups as adenoma, hyperplastic, other serrated lesions or others; APC and MutYH mutations were assessed. Mean age at diagnosis was 54 ± 14 years in men and 48 ± 13 in women (P = 0.005). Polyps were more numerous in women (37 ± 26 vs 29 ± 22). Sixty % of patients underwent bowel resection, mainly for cancer; the remaining were managed through endoscopy. A total of 2586 polyps were detected at diagnostic endoscopy: 2026 (80 %) were removed and analysed. Adenomas were diagnosed in 1445 (70 %), hyperplastic polyps in 541 (26 %), other serrated lesions in 61 (2.9 %). Adenomas and hyperplastic lesions were detected in the majority of patients. In 68 patients (81 %) in whom studies were executed, APC mutations were found in 8 and MutYH mutations in 10. Genetic variants were more frequent in women (12 vs 6, P = 0.039). Taking into consideration the prevalent (>50 %) histology and presence of mutations, patients could be subdivided into four groups: (1) APC mutated polyposis (AFAP), when adenomas were >50 % and APC mutations detected (no. 8, 10 %); (2) MutYH mutated polyposis (MAP), adenomas >50 % and biallelic MutYH mutations (no. 10, 12 %); (1) attenuated polyposis without detectable mutations, prevalence of adenomas, 48 cases (57 %); (1) hyperplastic-serrated polyposis, with prevalence (>50 %) of hyperplastic/other serrated lesions and no constitutional mutation (no. 18, 21 %). Aggregation of tumors, cancer in probands, distribution of polyps and other clinical characteristics showed no difference among the four groups. In conclusions, AFAP and MAP, the polyposis labeled by constitutional mutations, represented about 25 % of all attenuated polyposis. Mutation-associated cases showed an earlier age of onset of polyps and were more frequent in the female sex.

Similar content being viewed by others
References
Scott RJ (2003) Familial adenomatous polyposis (FAP) and other polyposis syndromes. Hered Cancer Clin Pract 1:19–30
Galiatsatos P, Foulkes WD (2006) Familial adenomatous polyposis. Am J Gastroenterol 101:385–398
Wäsch R, Robbins JA, Cross FR (2010) The emerging role of APC/CCdh1 in controlling differentiation, genomic stability and tumor suppression. Oncogene 29:1–10
Pezzi A, Roncucci L, Benatti P, Sassatelli R, Varesco L, Di Gregorio C, Venesio T, Pedroni M, Maffei S, Reggiani Bonetti L, Borsi E, Ferrari M, Martella P, Rossi G, Ponz De Leon M (2009) Relative role of APC and MUTYH mutations in the pathogenesis of familial adenomatous polyposis. Scand J Gastroenterol 44:1092–1100
Vasen HF, Möslein G, Alonso A, Aretz S, Bernstein I, Bertario L, Blanco I, Bülow S, Burn J, Capella G, Colas C, Engel C, Frayling I, Friedl W, Hes FJ, Hodgson S, Järvinen H, Mecklin JP, Møller P, Myrhøi T, Nagengast FM, Parc Y, Phillips R, Clark SK, de Leon MP, Renkonen-Sinisalo L, Sampson JR, Stormorken A, Tejpar S, Thomas HJ, Wijnen J (2008) Guidelines for the clinical management of familial adenomatous polyposis (FAP). Gut 57:704–713
Nielsen M, Hes FJ, Nagengast FM, Weiss MM, Mathus-Vliegen EM, Morreau H, Breuning MH, Wijnen JT, Tops CM, Vasen HF (2007) Germline mutations in APC and MUTYH are responsible for the majority of families with attenuated familial adenomatous polyposis. Clin Genet 71:427–433
Filipe B, Baltazar C, Albuquerque C, Fragoso S, Lage P, Vitoriano I, Mão de Ferro S, Claro I, Rodrigues P, Fidalgo P, Chaves P, Cravo M, Nobre Leitão C (2009) APC or MUTYH mutations account for the majority of clinically well-characterized families with FAP and AFAP phenotype and patients with more than 30 adenomas. Clin Genet 76:242–255
Wang L, Baudhuin LM, Boardman LA, Steenblock KJ, Petersen GM, Halling KC, French AJ, Johnson RA, Burgart LJ, Rabe K, Lindor NM, Thibodeau SN (2004) MYH mutations in patients with attenuated and classic polyposis and with young-onset colorectal cancer without polyps. Gastroenterology 127:9–16
Ponz de Leon M, Urso ED, Pucciarelli S, Agostini M, Nitti D, Roncucci L, Benatti P, Pedroni M, Kaleci S, Balsamo A, Laudi C, Di Gregorio C, Viel A, Rossi G, Venesio T (2013) Clinical and molecular features of attenuated adenomatous polyposis in northern Italy. Tech Coloproctol 17:79–87
Schlussel AT, Gagliano RA Jr, Seto-Donlon S, Eggerding F, Donlon T, Berenberg J, Lynch HT (2014) The evolution of colorectal cancer genetics-Part 2: clinical implications and applications. J Gastrointest Oncol 5:336–344
Shussman N, Wexner SD (2014) Colorectal polyps and polyposis syndromes. Gastroenterol Rep 2:1–15
Brosens LA, van Hattem A, Hylind LM, Iacobuzio-Donahue C, Romans KE, Axilbund J, Cruz-Correa M, Tersmette AC, Offerhaus GJ, Giardiello FM (2007) Risk of colorectal cancer in juvenile polyposis. Gut 56:965–967
Gill P, Wang LM, Bailey A, East JE, Leedham S, Chetty R (2013) Reporting trends of right-sided hyperplastic and sessile serrated polyps in a large teaching hospital over a 4-year period (2009–2012). J Clin Pathol 66:655–658
Mongin C, Coulet F, Lefevre JH, Colas C, Svrcek M, Eyries M, Lahely Y, Fléjou JF, Soubrier F, Parc Y (2012) Unexplained polyposis: a challenge for geneticists, pathologists and gastroenterologists. Clin Genet 81:38–46
Hazewinkel Y, Tytgat KM, van Eeden S, Bastiaansen B, Tanis PJ, Boparai KS, Fockens P, Dekker E (2014) Incidence of colonic neoplasia in patients with serrated polyposis syndrome who undergo annual endoscopic surveillance. Gastroenterology 147:88–95
Chetty R, Hafezi-Bakhtiari S, Serra S, Colling R, Wang LM (2015) Traditional serrated adenomas (TSAs) admixed with other serrated (so-called precursor) polyps and conventional adenomas: a frequent occurrence. J Clin Pathol 68:270–273
ICD-O, Classificazione Internazionale delle malattie, Oncologia, WHO (1983) A cura di F. Rilke. Istocitopatologia 5
Ponz de Leon M (2014) What clinicians wish to know about benign colorectal polyps: an operative classification. Pathol Res Pract 210:645–648
Ponz de Leon M, Benatti P, Percesepe A, Cacciatore A, Sassatelli R, Bertoni G, Sabadini G, Varesco L, Gismondi V, Mareni C, Montera M, Di Gregorio C, Landi P, Roncucci L (1999) Clinical features and genotype-phenotype correlations in 41 Italian families with adenomatosis coli. Ital J Gastroenterol Hepatol 31:850–860
Venesio T, Balsamo A, Sfiligoi C, Fuso L, Molatore S, Ranzani GN, Risio M (2007) Constitutional high expression of an APC mRNA isoform in a subset of attenuated familial adenomatous polyposis patients. J Mol Med 85:305–312
Venesio T, Molatore S, Cattaneo F, Arrigoni A, Risio M, Ranzani GN (2004) High frequency of MYH gene mutations in a subset of patients with familial adenomatous polyposis. Gastroenterology 126:1681–1685
Nielsen M, Joerink-van de Beld MC, Jones N, Vogt S, Tops CM, Vasen HF, Sampson JR, Aretz S, Hes FJ (2009) Analysis of MUTYH genotypes and colorectal phenotypes in patients With MUTYH-associated polyposis. Gastroenterology 136:471–476
Burt RW, Jass J (2000) Hyperplastic polyposis. In: Hamilton SR, Aaltonen LA (eds) World Health Organization classification of tumours pathology and genetics. Springer, Berlin, pp 135–136
Spring KJ, Zhao ZZ, Karamatic R, Walsh MD, Whitehall VL, Pike T, Simms LA, Young J, James M, Montgomery GW, Appleyard M, Hewett D, Togashi K, Jass JR, Leggett BA (2006) High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology 131:1400–1407
Kambara T, Simms LA, Whitehall VL, Spring KJ, Wynter CV, Walsh MD, Barker MA, Arnold S, McGivern A, Matsubara N, Tanaka N, Higuchi T, Young J, Jass JR, Leggett BA (2004) BRAF mutation is associated with DNA methylation in serrated polyps and cancers of the colorectum. Gut 53:1137–1144
Iino H, Simms L, Young J, Arnold J, Winship IM, Webb SI, Furlong KL, Leggett B, Jass JR (2000) DNA microsatellite instability and mismatch repair protein loss in adenomas presenting in hereditary non-polyposis colorectal cancer. Gut 47:37–42
Ngeow J, Heald B, Rybicki LA, Orloff MS, Chen JL, Liu X, Yerian L, Willis J, Lehtonen HJ, Lehtonen R, Mester JL, Moline J, Burke CA, Church J, Aaltonen LA, Eng C (2013) Prevalence of germline PTEN, BMPR1A, SMAD4, STK11, and ENG mutations in patients with moderate-load colorectal polyps. Gastroenterology 144:1402–1409
Palles C, Cazier JB, Howarth KM, Domingo E, Jones AM, Broderick P, Kemp Z, Spain SL, Guarino E, Salguero I, Sherborne A, Chubb D, Carvajal-Carmona LG, Ma Y, Kaur K, Dobbins S, Barclay E, Gorman M, Martin L, Kovac MB, Humphray S; CORGI Consortium; WGS500 Consortium, Lucassen A, Holmes CC, Bentley D, Donnelly P, Taylor J, Petridis C, Roylance R, Sawyer EJ, Kerr DJ, Clark S, Grimes J, Kearsey SE, Thomas HJ, McVean G, Houlston RS, Tomlinson I (2013) Germline mutations affecting the proofreading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nat Genet 45:136–144
Vasen HFA, Tomlinson I, Castells A (2015) Clinical management of hereditary colorectal cancer syndromes. Nat Rev Gastroenterol Hepatol 12:88–97
Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW, American College of Gastroenterology (2015) ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol 110:223–262
Lubbe SJ, Di Bernardo MC, Chandler IP, Houlston RS (2009) Clinical implications of the colorectal cancer risk associated with MUTYH mutation. J Clin Oncol 27:3975–3980
Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC (1995) Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med 1(122):327–334
Wallace K, Baron JA, Karagas MR, Cole BF, Byers T, Beach MA, Pearson LH, Burke CA, Silverman WB, Sandler RS (2005) The association of physical activity and body mass index with the risk of large bowel polyps. Cancer Epidemiol Biomarkers Prev 14:2082–2086
Boparai KS, van den Broek FJ, van Eeden S, Fockens P, Dekker E (2011) Increased polyp detection using narrow band imaging compared with high resolution endoscopy in patients with hyperplastic polyposis syndrome. Endoscopy 43:676–682
Acknowledgments
The Authors wish to thank ARTI (Associazione Ricerca Tumori Intestinali, Modena, Italy) for grants to Dr. G. Rossi and G. Magnani. The paper has been presented at the Annual Meeting of the Italian Society of Pathology (SIAPEC, Milan, Italy, 23–25 September 2015) and at the Annual Meeting of the Italian Association for the study of Hereditary Digestive Tumors (AIFEG, Naples, Italy, 12–13 November 2015).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Leon, M.P., Pedroni, M., Roncucci, L. et al. Attenuated polyposis of the large bowel: a morphologic and molecular approach. Familial Cancer 16, 211–220 (2017). https://doi.org/10.1007/s10689-016-9938-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10689-016-9938-9