Abstract
Approximately 15 % of colorectal carcinomas (CRC) display high level microsatellite instability (MSI-H) due to either a germline mutation in one of the genes responsible for DNA mismatch repair (Lynch syndrome, 3 %) or somatic inactivation of the same pathway, most commonly through hypermethylation of the MLH1 gene (sporadic MSI-H, 12 %). Although heterogeneous, MSI-H colorectal carcinomas as a group show some distinct biologic characteristics when compared to CRC with stable or low level microsatellite instability. In the present review we will highlight therapeutically relevant characteristics of MSI-H tumors which could lead to specific responses to some conventional chemotherapy or novel targeted therapy agents.
Similar content being viewed by others
Introduction
Colorectal carcinoma (CRC) represents the third most common malignancy in the developed world and one of the leading causes of cancer-related death [1]. At the molecular level, CRC is a heterogeneous disease with several molecular subtypes that harbor distinct molecular genetic, pathologic and clinical characteristics [2]. Recently, the consensus molecular subtypes (CMS) of CRC have been defined [3]. According to the new classification, four CMS with distinguishing characteristics have been proposed: CMS1 (microsatellite instability immune subtype: hypermutated subtype of CRC, microsatellite unstable with a strong immune activation); CMS2 (canonical subtype of CRC: epithelial subtype with upregulation of the WNT and MYC signaling pathways); CMS3 (metabolic subtype of CRC: epithelial subtype with metabolic dysregulation); and CMS4 [mesenchymal subtype of CRC with prominent transforming growth factor-β (TGF-β) activation, stromal invasion and neoangiogenesis] [3].
MSI refers to the hypermutable state of cells caused by impaired DNA mismatch repair (MMR). It consists of insertion and deletion mutations in stretches of short tandem DNA repeats (microsatellites) as well as nucleotide substitutions throughout the genome [4]. In this review, we simplify classification of molecular subtypes of CRC based on MSI status into two broad subgroups: MSI-high (MSI-H) and MSI-negative (low or stable) CRCs, in an effort to highlight potential therapeutic differences between these easily separable groups.
MSI-high (MSI-H) colorectal cancer
MSI-H CRC accounts for 15 % of all CRC and includes hereditary non-polyposis colorectal cancer (HNPCC) or Lynch syndrome (3 %) and sporadic MSI-H CRC (12 %). Lynch syndrome is a highly penetrant (80 % life time risk for CRC), autosomal-dominant disorder caused by germline mutations in one of the MMR genes: MLH1, MSH2 (70 %), MSH6 and PMS2 (~30 %) [5, 6] (Fig. 1A, B). In addition, germline deletions of the last exon of the epithelial cell adhesion molecule [(EPCAM), a gene located upstream of MSH2] cause Lynch syndrome via epigenetic inactivation of MSH2 [7].
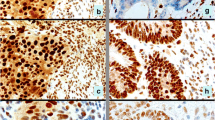
A colorectal carcinoma from a case of Lynch syndrome caused by an MLH1 gene mutation: A hematoxylin and eosin (H&E) stained slide, B immunohistochemistry (IHC) showing concurrent loss of PMS2 in tumor cells, C tumor cells were diffusely positive (90–100 %) for topoisomerase 1 and D strongly positive (3+) for thymidylate synthase
Sporadic MSI-H CRCs are typically caused by somatic methylation of the MLH1 gene promoter [4] (Fig. 2A, B). It is worth noting that a small subset of MSI-H tumors harbor no alterations in the MMR genes, but overexpress various miRNAs that may silence the MMR genes. Thus, miRNA-155 downregulates MLH1, MSH2 and MSH6 mRNA, inducing MSI in CRC cell lines [8]. Similarly, miRNA-21, targeting MSH2 and MSH6 mRNA, has been found to be overexpressed in MSI-H CRC [9]. In addition, Li et al. [10, 11] found that cells lacking the SETD2 histone methyltransferase displayed microsatellite instability.
A poorly differentiated (signet ring) colorectal carcinoma with microsatellite instability-high status caused by the loss of MLH1: A H&E-stained slide, B loss of MLH1 in tumor cells by IHC, C concurrent loss of PMS2 in tumor cells by IHC; note retained expression of both MLH1 and PMS2 proteins in adjacent tumor-infiltrating lymphocytes, D IHC showing that the tumor also harbored the BRAF V600E mutation, E the tumor cells exhibited 2+ PD-L1 expression in ~85 % of the tumor cells (anti-PD-L1 clone SP142) and F while tumor infiltrating lymphocytes were positive for PD-1 protein
Regardless of the origin (hereditary or sporadic) or type of mutation, MSI-H CRCs share some distinct histologic cancer features (mucin-rich, signet ring and medullary types, often admixed) with increased numbers of tumor-infiltrating lymphocytes (TILs) and prominent Crohn’s-like lymphoid reaction [6, 12]. In addition, patients with Lynch syndrome have an increased risk of synchronous or metachronous tumors that include extracolonic sites (small bowel, stomach, endometrium, skin, genitourinary tract) [5, 13]. Prognostically, patients with HNPCC have a more favorable outcome (overall survival) in comparison with stage-matched sporadic CRCs [14, 15].
Methylation of the MLH1 promoter region that is typically seen in sporadic MSI-H CRC, but not in Lynch syndrome, is strongly associated with the BRAF V600E gene mutation [16, 17] (Fig. 2D). In fact, presence of the BRAF V600E mutation in CRC essentially excludes Lynch syndrome, with the exception of rare cases associated with PMS2 germline mutation [18, 19].
MSI-H colorectal cancers in the era of personalized medicine
CRC is the second leading cause of cancer-related death in the developed world, [20]. Although the response rate of metastatic CRC to the combined chemotherapy is around 50 %, progression of the disease is inevitable and less than 10 % of patients survive >2 years [20]. In adjunct to conventional chemotherapy (e.g. 5-FU, capecitabine, oxaliplatin, irinotecan), metastatic CRC is now treated with a number of drugs aimed at target-specific signaling pathways [e.g. anti-EGFR based therapy (panitumumab and cetuximab for KRAS/NRAS wild type CRC); bevacizumab (for inhibition of angiogenesis)] [20, 21]. There is an urgent need for more specific predictive markers that will tailor the CRC treatment modalities and improve overall survival in patients with locally advanced and/or metastatic disease.
Predictive biomarkers of conventional chemotherapy
MSI-H status due to loss of MMR gene function is not only a key player in the pathogenesis of CRC, but is also associated with a different response to classic chemotherapeutic treatment modalities [6].
A seminal clinical study by Ribic et al. [15] revealed the benefit of 5-FU-based adjuvant chemotherapy in patients with stage II and stage III MSI-negative CRC (HR = 0.72, p = 0.04) but not in those with MSI-H status (HR = 1.07, p = 0.80). Preclinical data also confirmed that tumor cells with MSI-H status are resistant to fluoropyrimidines [e.g. 5-fluorouracil (5-FU) and capecitabine], but may be sensitive to irinotecan and mitomycin C [4, 6, 21]. A meta-analysis by Guetz et al. [22] also highlighted MSI-H status as a strong predictive factor for non-response to 5-FU based chemotherapy. Several enzymes including dihydropyrimidine dehydrogenase (DPD), orotate phosphoribosyl transferase (OPRT), thymidine phosphorylase (TP) and thymidylate synthase (TS) have been associated with the metabolism of the 5-FU pathway [21]. TS is a key enzyme involved in the synthesis of 2′-deoxythymidine-5′-monophosphate, which represents an essential precursor for DNA synthesis [23]. Although data from the available literature are not consistent [(different methodologies, cutoff values), reviewed in Koopman et al.], several studies have found significantly higher expression of TS in MSI-H CRCs, including Lynch syndrome cases, and an association with resistance to 5-FU chemotherapy regimen [24–26] (Fig. 1D). In contrast, MSI-H CRCs appear to be more sensitive to irinotecan which functions as an inhibitor of topoisomerase 1 (TOP1) [4]. Colorectal cancer cell lines exhibit sensitivity to irinotecan when harboring increased TOP1 gene copy number or increased TOP1/CEP20 ratio [27]. Topoisomerase 1 protein overexpression has also been described in MSI-H CRC [28], although Søndenstrup et al. [29] recently reported an absence of TOP1 gene copy number gain.
Our results, based on the analysis of both sporadic and hereditary MSI-H and MSI-negative CRCs support the reported differences in TS protein [30] (Fig. 1C; Table 1). TS expression was significantly higher in MSI-H tumors, both sporadic (86 %) and hereditary (100 %), compared to an MSI-negative cohort (31 %, p < 0.0001) (Table 1; Fig. 1D). Protein expression of TOP1 trends higher in the Lynch cohort, but is indistinguishable between the sporadic MSI-H and MSI-negative cohorts [30, 31] (Table 1; Fig. 1C).
Another biomarker which has been associated with MSI-H CRC is O6-methylguanine DNA methyltransferase (MGMT). MGMT is a DNA repair protein with the ability to remove various carcinogenic adducts from the O6 position of guanine [32, 33]. Aberrant methylation of the MGMT gene promoter occurs in CRCs with the CpG island methylator phenotype (CIMP) [34], and this correlates with loss of MGMT expression [32]. In CRC, MGMT hypermethylation has been described in subsets of both sporadic and hereditary MSI-H tumors [32, 35, 36] (Fig. 3A, B).
In our cohort of CRC tumors, MGMT protein expression was much lower (33 %) in the sporadic MSI-H cohort compared to MSI-H tumors without BRAF mutation (54 %); this difference may be attributable to CIMP in the BRAF mutant tumors (Table 1).
MGMT has also been shown to serve as a predictor of response to alkylating agents (temozolomide and dacarbazine) that have been approved for the treatment of various cancers including brain tumors (astrocytoma and glioblastoma multiforme), melanoma, sarcoma, and Hodgkin lymphoma [26, 37]. In colorectal cancer, temozolomide showed limited clinical activity in unselected patient cohorts, but when patients were selected for low expression of MGMT, very promising results were seen [32, 37–40]. However, Karran in his comment [41] pointed out the importance of defects within the mismatch repair machinery in cancer and potential resistance to various chemotherapeutics, including alkylating drugs. In the context of MSI-H CRC and alkylating agents, it is worth noting a study by Hunter et al. [42] who confirmed that inactivating somatic mutations of the MSH6 gene not only confer a resistance to alkylating agents in brain tumors (gliomas) but also promote tumor growth and progression.
Next generation sequencing (NGS) profiling in MSI-H
Studies using the currently available NGS platforms allow for investigation into molecular pathways known to contribute to tumorigenesis and progression of CRC and their differential contributions in the setting of sporadic and germline MSI-H CRCs. Several studies reported a frequent disruption of the WNT signaling pathway in Lynch syndrome, with mutations commonly affecting the APC and CTNNB1 (beta-catenin) genes, which are less commonly mutated in sporadic MSI-H CRC [43]. Recent comprehensive molecular profiling of CRC using whole-genome sequencing revealed that the majority (75 %) of hypermutated CRCs exhibited MSI-H status, associated with hypermethylation of MLH1, while the remaining 25 % had somatic mismatch-repair gene and polymerase ε (POLE) mutations [44]. Along with the expected mutations of APC, TP53, SMAD4, PIK3CA and KRAS, the study revealed frequent mutations in ARID1A, SOX9 and FAM123B genes, while copy-number alterations included amplification of the ERBB2 and IGF2 genes. Integrative analysis also indicated an important role for MYC-directed transcriptional activation and repression [44]. Timmermann et al. [45] also reported a significantly higher incidence of mutations in MSI-H than in MSI-negative CRCs.
Le et al. [46] used whole-exome sequencing and showed a mean of 1782 somatic mutations per tumor in patients with mismatch repair-deficient cancer (N = 9) as compared with 73 mutations per tumor in patients with mismatch repair-proficient cancer (N = 6) (p = 0.007). Similarly, we performed NGS using a more limited 591-gene panel (available here: http://www.carismolecularintelligence.com/) on 8 MSI-H CRCs, and observed an average of 130.25 mutations per tumor compared to an average of 55.17 mutations per tumor in 189 MSI-negative CRCs (p = 0.0004, Student’s t test, unpublished data).
Immune checkpoint proteins PD-1 and PD-L1 in MSI-H colorectal cancer
The PD-1 signaling pathway, composed of the immune cell co-receptor Programmed Death 1 (PDCD1, CD279) and its ligands PD-L1 (B7-H1, CD274) and PD-L2 (PDCD1LG2, B7-DC, CD273), is actively involved in local immunosuppression in human tumors [47]. PD-L1 expression in tumor and associated inflammatory cells has been described in different malignancies, correlating with poor clinical outcome but also with the likelihood of response to targeted immune check point inhibition therapy [48–52]. Several therapeutic monoclonal antibodies inhibiting either PD-1 (nivolumab, pembrolizumab) or PD-L1 (MPDL3280A, Medi4736, BMS-936559) have been developed and are now used for the treatment of various malignancies (e.g. metastatic melanoma, non-small cell lung carcinoma, renal cell carcinoma, bladder carcinoma and Hodgkin lymphoma) [52–54].
In contrast to MSI-negative CRCs, MSI-H CRCs exhibit an active immune microenvironment infiltrated by cytotoxic (CD8+) T-lymphocytes and activated Th1 cells characterized by interferon-γ production and the Th1 transcription factor TBET. This response likely results from the presence of numerous neoantigens (mutated proteins) resulting from the hyper-mutated state of the tumor cells [46, 55–57]. Despite such a “hostile” microenvironment, MSI-H tumor cells are not eliminated by the immune system due to the cancer specific upregulation of various immune inhibitory molecules (checkpoints) including PD-1, PD-L1, Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), Lymphocyte-activation gene 3 (LAG-3), and Indoleamine (2,3)-dioxygenase (IDO) [31, 55, 58] (Fig. 2E, F; Table 1). These data indicate that MSI-H CRCs are good candidates for checkpoint immunotherapy as recently shown in a small phase 2 clinical trial which included 11 patients with MSI-H CRC. The study showed that the immune-related objective response and immune-related progression-free survival rates were 40 and 78 %, respectively for refractory/and metastatic MSI-H CRC in contrast to MSI-negative CRC (0 and 11 %, respectively) [46]. This led the US Food and Drug Administration to rapidly approve the anti-PD-1 drug pembrolizumab for the treatment of metastatic/refractory MSI-H CRC. Additional studies and clinical trials involving more samples/patients should define the optimal predictive biomarkers for immune checkpoint inhibitors as well as their therapeutic benefits for patients with MSI-H CRC [53].
Conclusions
Although MSI-H colorectal cancers are heterogeneous (i.e. they can be caused by germline or somatic mutations in different genes), they have similar characteristics that allow them to be grouped together for treatment and clinical management. MSI-H CRCs respond poorly to 5-FU-based chemotherapy (based on thymidylate synthase overexpression), but they may be efficiently treated with camptothecin derivatives (based on topoisomerase 1 overexpression). Hypermethylation/loss of expression of MGMT, which differs between MSI-H cancers based on underlying pathogenic mechanism (i.e. sporadic CIMP MSI-H CRC will be expected to have significantly different MGMT expression from hereditary Lynch MSI-H CRC), may identify a patient subset for clinical investigation. These findings point to the need for individualized profiling of biomarkers and tailoring of therapy for CRCs. Due to an active immune microenvironment and high expression of various checkpoint molecules, MSI-H CRCs are good candidates for targeted immunotherapy with immune checkpoint inhibitors (e.g. pembrolizumab). These cancers also exhibit a distinct hyper-mutated profile that may be amenable to additional treatment options such as vaccination and adoptive-cell-transfer therapy.
References
Siegel RL, Miller KD, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65(1):5–29. doi:10.3322/caac.21254
Dienstmann R, Salazar R, Tabernero J (2014) The evolution of our molecular understanding of colorectal cancer: what we are doing now, what the future holds, and how tumor profiling is just the beginning. Am Soc Clin Oncol Educ Book. doi:10.14694/EdBook_AM.2014.34.91
Guinney J, Dienstmann R, Wang X et al (2015) The consensus molecular subtypes of colorectal cancer. Nat Med 21(11):1350–1356. doi:10.1038/nm.3967
Devaud N, Gallinger S (2013) Chemotherapy of MMR-deficient colorectal cancer. Fam Cancer 12(2):301–306. doi:10.1007/s10689-013-9633-z
Cheng L (2009) Molecular genetic pathology, 1st edn. Humana Press, New Jersey, pp 455–457.
Hewish M, Lord CJ, Martin SA, Cunningham D, Ashworth A (2010) Mismatch repair deficient colorectal cancer in the era of personalized treatment. Nat Rev Clin Oncol 7(4):197–208. doi:10.1038/nrclinonc.2010.18
Ligtenberg MJ, Kuiper RP, Chan TL et al (2009) Heritable somatic methylation and inactivation of MSH2 in families with Lynch syndrome due to deletion of the 3′ exons of TACSTD1. Nat Genet 41(1):112–117. doi:10.1038/ng.283
Valeri N, Gasparini P, Fabbri M et al (2010) Modulation of mismatch repair and genomic stability by miR-155. Proc Natl Acad Sci USA 107(15):6982–6987. doi:10.1073/pnas.1002472107
Volinia S, Calin GA, Liu CG et al (2006) A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci USA 103(7):2257–2261. doi:10.1073/pnas.0510565103
Li GM (2013) Decoding the histone code: role of H3K36me3 in mismatch repair and implications for cancer susceptibility and therapy. Cancer Res 73(21):6379–6383. doi:10.1158/0008-5472.can-13-1870
Li F, Mao G, Tong D et al (2013) The histone mark H3K36me3 regulates human DNA mismatch repair through its interaction with MutSα. Cell 153(3):590–600. doi:10.1016/j.cell.2013.03.025
Smyrk TC, Watson P, Kaul K, Lynch HT (2001) Tumor-infiltrating lymphocytes are a marker for microsatellite instability in colorectal carcinoma. Cancer 91(12):2417–2422
Gatalica Z, Torlakovic E (2008) Pathology of the hereditary colorectal carcinoma. Fam Cancer 7(1):15–26. doi:10.1007/s10689-007-9146-8
Maccaroni E, Bracci R, Giampieri R et al (2015) Prognostic impact of mismatch repair genes germline defects in colorectal cancer patients: are all mutations equal? Oncotarget 6(36):38737–38748. doi:10.18632/oncotarget.5395
Ribic CM, Sargent DJ, Moore MJ et al (2003) Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 349(3):247–257. doi:10.1056/NEJMoa022289
Weisenberger DJ, Siegmund KD, Campan M et al (2006) CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nat Genet 38(7):787–793. doi:10.1038/ng1834
Parsons MT, Buchanan DD, Thompson B, Young JP, Spurdle AB (2012) Correlation of tumour BRAF mutations and MLH1 methylation with germline mismatch repair (MMR) gene mutation status: a literature review assessing utility of tumour features for MMR variant classification. J Med Genet 49(3):151–157. doi:10.1136/jmedgenet-2011-100714
Funkhouser WK Jr, Lubin IM, Monzon FA et al (2012) Relevance, pathogenesis, and testing algorithm for mismatch repair-defective colorectal carcinomas: a report of the association for molecular pathology. J Mol Diagn JMD 14(2):91–103. doi:10.1016/j.jmoldx.2011.11.001
Senter L, Clendenning M, Sotamaa K et al (2008) The clinical phenotype of Lynch syndrome due to germ-line PMS2 mutations. Gastroenterology 135(2):419–428. doi:10.1053/j.gastro.2008.04.026
Maurel J, Postigo A (2015) Prognostic and predictive biomarkers in colorectal cancer. From the preclinical setting to clinical practice. Curr Cancer Drug Targets 15(8):703–715
Koopman M, Venderbosch S, van Tinteren H et al (2009) Predictive and prognostic markers for the outcome of chemotherapy in advanced colorectal cancer, a retrospective analysis of the phase III randomised CAIRO study. Eur J Cancer 45(11):1999–2006. doi:10.1016/j.ejca.2009.04.017
Des Guetz G, Schischmanoff O, Nicolas P, Perret GY, Morere JF, Uzzan B (2009) Does microsatellite instability predict the efficacy of adjuvant chemotherapy in colorectal cancer? A systematic review with meta-analysis. Eur J Cancer 45(10):1890–1896. doi:10.1016/j.ejca.2009.04.018
Rose MG, Farrell MP, Schmitz JC (2002) Thymidylate synthase: a critical target for cancer chemotherapy. Clin Colorectal Cancer 1(4):220–229. doi:10.3816/CCC.2002.n.003
Jensen SA, Vainer B, Kruhoffer M, Sorensen JB (2009) Microsatellite instability in colorectal cancer and association with thymidylate synthase and dihydropyrimidine dehydrogenase expression. BMC Cancer 9:25. doi:10.1186/1471-2407-9-25
Bendardaf R, Lamlum H, Ristamaki R, Korkeila E, Syrjanen K, Pyrhonen S (2008) Thymidylate synthase and microsatellite instability in colorectal cancer: implications for disease free survival, treatment response and survival with metastases. Acta Oncol 47(6):1046–1053. doi:10.1080/02841860701678753
Coppede F, Lopomo A, Spisni R, Migliore L (2014) Genetic and epigenetic biomarkers for diagnosis, prognosis and treatment of colorectal cancer. World J Gastroenterol 20(4):943–956. doi:10.3748/wjg.v20.i4.943
Romer MU, Jensen NF, Nielsen SL et al (2012) TOP1 gene copy numbers in colorectal cancer samples and cell lines and their association to in vitro drug sensitivity. Scand J Gastroenterol 47(1):68–79. doi:10.3109/00365521.2011.638393
Negri FV, Azzoni C, Bottarelli L et al (2013) Thymidylate synthase, topoisomerase-1 and microsatellite instability: relationship with outcome in mucinous colorectal cancer treated with fluorouracil. Anticancer Res 33(10):4611–4617
Sonderstrup IM, Nygard SB, Poulsen TS et al (2015) Topoisomerase-1 and -2A gene copy numbers are elevated in mismatch repair-proficient colorectal cancers. Mol Oncol 9(6):1207–1217. doi:10.1016/j.molonc.2015.02.009
Gatalica Z (2014) Thymidylate synthase over-expression underlies the observed lack of 5-FU therapy benefit for MSI-H colorectal cancers. Ann Oncol 25(suppl 4):iv167–iv209
Gatalica Z, Vijayvergia N, Vranic S, Xiu J, Reddy S, Lynch HT, El-Deiry WS (2015) Therapeutic biomarker differences between MSI-H and MSS colorectal cancers. J Clin Oncol 33(Suppl; abstr 3597)
Zheng CG, Jin C, Ye LC, Chen NZ, Chen ZJ (2015) Clinicopathological significance and potential drug target of O6-methylguanine-DNA methyltransferase in colorectal cancer: a meta-analysis. Tumour Biol 36(8):5839–5848. doi:10.1007/s13277-015-3254-0
Kaina B, Christmann M, Naumann S, Roos WP (2007) MGMT: key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA Repair 6(8):1079–1099. doi:10.1016/j.dnarep.2007.03.008
Nazemalhosseini Mojarad E, Kuppen PJ, Aghdaei HA, Zali MR (2013) The CpG island methylator phenotype (CIMP) in colorectal cancer. Gastroenterol Hepatol Bed Bench 6(3):120–128
Gonzalo V, Lozano JJ, Munoz J et al (2010) Aberrant gene promoter methylation associated with sporadic multiple colorectal cancer. PLoS One 5(1):e8777. doi:10.1371/journal.pone.0008777
Kaz A, Kim YH, Dzieciatkowski S et al (2007) Evidence for the role of aberrant DNA methylation in the pathogenesis of Lynch syndrome adenomas. Int J Cancer 120(9):1922–1929. doi:10.1002/ijc.22544
Inno A, Fanetti G, Di Bartolomeo M et al (2014) Role of MGMT as biomarker in colorectal cancer. World J Clin Cases 2(12):835–839. doi:10.12998/wjcc.v2.i12.835
Shacham-Shmueli E, Beny A, Geva R, Blachar A, Figer A, Aderka D (2011) Response to temozolomide in patients with metastatic colorectal cancer with loss of MGMT expression: a new approach in the era of personalized medicine? J Clin Oncol 29(10):e262–e265. doi:10.1200/jco.2010.32.0242
Pietrantonio F, de Braud F, Milione M et al (2015) Dose-dense temozolomide in patients with MGMT-silenced chemorefractory colorectal cancer. DOI, Target Oncol. doi:10.1007/s11523-015-0397-2
Li Y, Lyu Z, Zhao L et al (2015) Prognostic value of MGMT methylation in colorectal cancer: a meta-analysis and literature review. Tumour Biol 36(3):1595–1601. doi:10.1007/s13277-014-2752-9
Karran P (2001) Mechanisms of tolerance to DNA damaging therapeutic drugs. Carcinogenesis 22(12):1931–1937
Hunter C, Smith R, Cahill DP et al (2006) A hypermutation phenotype and somatic MSH6 mutations in recurrent human malignant gliomas after alkylator chemotherapy. Cancer Res 66(8):3987–3991. doi:10.1158/0008-5472.CAN-06-0127
Dionigi G, Bianchi V, Villa F et al (2007) Differences between familial and sporadic forms of colorectal cancer with DNA microsatellite instability. Surg Oncol 16(Suppl 1):S37–S42. doi:10.1016/j.suronc.2007.10.018
TCGA (2012) Comprehensive molecular characterization of human colon and rectal cancer. Nature 487(7407):330–337. doi:10.1038/nature11252
Timmermann B, Kerick M, Roehr C et al (2010) Somatic mutation profiles of MSI and MSS colorectal cancer identified by whole exome next generation sequencing and bioinformatics analysis. PLoS One 5(12):e15661. doi:10.1371/journal.pone.0015661
Le DT, Uram JN, Wang H et al (2015) PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 372(26):2509–2520. doi:10.1056/NEJMoa1500596
Lipson EJ, Forde PM, Hammers HJ, Emens LA, Taube JM, Topalian SL (2015) Antagonists of PD-1 and PD-L1 in cancer treatment. Semin Oncol 42(4):587–600. doi:10.1053/j.seminoncol.2015.05.013
Herbst RS, Soria JC, Kowanetz M et al (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515(7528):563–567. doi:10.1038/nature14011
Taube JM, Klein A, Brahmer JR et al (2014) Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 20(19):5064–5074. doi:10.1158/1078-0432.ccr-13-3271
Taube JM, Young GD, McMiller TL et al (2015) Differential expression of immune-regulatory genes associated with PD-L1 display in melanoma: implications for PD-1 pathway blockade. Clin Cancer Res 21(17):3969–3976. doi:10.1158/1078-0432.ccr-15-0244
Taube JM (2014) Unleashing the immune system: PD-1 and PD-Ls in the pre-treatment tumor microenvironment and correlation with response to PD-1/PD-L1 blockade. Oncoimmunology 3(11):e963413. doi:10.4161/21624011.2014.963413
Tumeh PC, Harview CL, Yearley JH et al (2014) PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515(7528):568–571. doi:10.1038/nature13954
de Guillebon E, Roussille P, Frouin E, Tougeron D (2015) Anti program death-1/anti program death-ligand 1 in digestive cancers. World J Gastrointest Oncol 7(8):95–101. doi:10.4251/wjgo.v7.i8.95
Sunshine J, Taube JM (2015) PD-1/PD-L1 inhibitors. Curr Opin Pharmacol 23:32–38. doi:10.1016/j.coph.2015.05.011
Llosa NJ, Cruise M, Tam A et al (2015) The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov 5(1):43–51. doi:10.1158/2159-8290.cd-14-0863
Topalian SL, Hodi FS, Brahmer JR et al (2012) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366(26):2443–2454. doi:10.1056/NEJMoa1200690
Yamamoto H, Imai K (2015) Microsatellite instability: an update. Arch Toxicol 89(6):899–921. doi:10.1007/s00204-015-1474-0
Gatalica Z, Snyder C, Maney T et al (2014) Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomark Prev 23(12):2965–2970. doi:10.1158/1055-9965.epi-14-0654
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Zoran Gatalica, Joanne Xiu, Jeffrey Swensen, and Sandeep Reddy are employees of Caris Life Sciences. Semir Vranic has received honoraria from Caris Life Sciences.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gatalica, Z., Vranic, S., Xiu, J. et al. High microsatellite instability (MSI-H) colorectal carcinoma: a brief review of predictive biomarkers in the era of personalized medicine. Familial Cancer 15, 405–412 (2016). https://doi.org/10.1007/s10689-016-9884-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10689-016-9884-6