Abstract
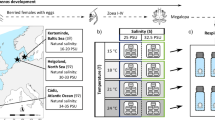
Both phenotypic plasticity and local genetic adaptation may contribute to a species’ ability to inhabit different environmental conditions. While phenotypic plasticity is usually considered costly, local adaptation takes generations to respond to environmental change and may be constrained by strong gene flow. The majority of marine species have complex life-cycles with pelagic stages that might be expected to promote gene flow and plastic responses, and yet several notable examples of local adaptation have been found in species with broadcast larvae. In the ascidian, Ciona intestinalis (Linnaeus, 1767),—a common marine species with broadcast spawning and a short larval stage—previous studies have found marked differences in salinity tolerance of early life-history stages among populations from different salinity regimes. We used common-garden experiments to test whether observed differences in salinity tolerance could be explained by phenotypic plasticity. Adult ascidians from two low salinity populations [2–5 m depth, ~25 practical salinity units (PSU)], and two full salinity populations (25–27 m depth, ~31 PSU) were acclimated for 2–4 weeks at both 25 and 31 PSU. Gametes were fertilized at the acclimation salinities, and the newly formed embryos were transferred to 10 different salinities (21–39 PSU) and cultured to metamorphosis. Adult acclimation salinity had an overriding and significant effect on larval metamorphic success: tolerance norms for larvae almost fully matched the acclimation salinity of the parents, independent of parental origin (deep or shallow). However we also detected minor population differences that could be attributed to either local adaptation or persistent environmental effects. We conclude that differences in salinity tolerance of C. intestinalis larvae from different populations are driven primarily by transgenerational phenotypic plasticity, a strategy that seems particularly favourable for an organism living in coastal waters where salinity is less readily predicted than in the open oceans.



Similar content being viewed by others
Notes
i.e. when trait expression of the offspring is influenced by the parental environment through non-genetic parental effects.
References
Berrigan D, Scheiner SM (2003) Modeling the evolution of phenotypic plasticity. In: DeWitt TJ, Scheiner SM (eds) Phenotypic plasticity : functional and conceptual approaches. Oxford University Press, New York, pp 82–97
Cohen S (1990) Outcrossing in field populations of two species of self-fertile ascidians. J Exp Mar Biol Ecol 140(3):147–158
David JR, Gibert P, Moreteau B (2003) Evolution of reaction norms. In: Thomas J DeWitt SMS (ed) Phenotypic plasticity: functional and conceptual approaches. Oxford University Press, New York, pp 50–63
DeWitt TJ, Scheiner SM (2003) Phenotypic plasticity : functional and conceptual approaches. Oxford University Press, New York
Dupont L, Viard F, Dowell MJ, Wood C, Bishop JD (2009) Fine- and regional-scale genetic structure of the exotic ascidian Styela clava (Tunicata) in southwest England, 50 years after its introduction. Mol Ecol 18(3):442–453
Dybern BI (1967) The distribution and salinity tolerance of Ciona intestinalis (L.) f. typica with special reference to the waters around Southern Scandinavia. Ophelia 4(2):207–226
Galloway LF (2005) Maternal effects provide phenotypic adaptation to local environmental conditions. New Phytol 166(1):93–99
Grosholz E (2001) Small spatial-scale differentiation among populations of an introduced colonial invertebrate. Oecologia 129(1):58–64
Hadfield MG, Strathmann MF (1996) Variability, flexibility and plasticity in life histories of marine invertebrates. Oceanol Acta 19(3–4):323–334
Harvell CD (1998) Genetic variation and polymorphism in the inducible spines of a marine bryozoan. Evolution 52(1):80–86
Havenhand JN (1991) Fertilisation and the potential for dispersal of gametes and larvae in the solitary ascidian Ascidia mentula Müller. Ophelia 33(1):1–15
Hollander J, Collyer ML, Adams DC, Johannesson K (2006) Phenotypic plasticity in two marine snails: constraints superseding life history. 19(6):1861–1872
Hollander J (2008) Testing the grain-size model for the evolution of phenotypic plasticity. Evolution 62(6):1381–1389
Jablonski D (1986) Larval ecology and macroevolution in marine invertebrates. Bull Mar Sci 39(2):565–587
Johannesson K, André C (2006) Life on the margin: genetic isolation and diversity loss in a peripheral marine ecosystem, the Baltic Sea. Mol Ecol 15(8):2013–2029
Kawecki TJ (2008) Adaptation to marginal habitats. Ann Rev Ecol Evol Syst 39(1):321–342
Levins R (1968) Evolution in changing environments: some theoretical explorations. Princeton University Press, Princeton
Lind MI, Johansson F (2007) The degree of adaptive phenotypic plasticity is correlated with the spatial environmental heterogeneity experienced by island populations of Rana temporaria. J Evol Biol 20(4):1288–1297
Lind MI, Ingvarsson PK, Johansson H, Hall D, Johansson F (2011) Gene flow and selection on phenotypic plasticity in an island system of Rana temporaria. Evolution 65(3):684–697
Meyers LA, Bull JJ (2002) Fighting change with change: adaptive variation in an uncertain world. Trends Ecol Evol 17(12):551–557
Muth NZ, Pigliucci M (2007) Implementation of a novel framework for assessing species plasticity in biological invasions: responses of Centaurea and Crepis to phosphorus and water availability. J Ecol 95(5):1001–1013
Newlon AW, Yund PO, Stewart-Savage J (2003) Phenotypic plasticity of reproductive effort in a colonial ascidian Botryllus schlosseri. J Exp Zool 297A(2):180–188
Nomaguchi TA, Nishijima C, Minowa S, Hashimoto M, Haraguchi C, Amemiya S, Fujisawa H (1997) Embryonic thermosensitivity of the ascidian Ciona savignyi. Zool Sci 14(3):511–515
Piersma T, Drent JA (2003) Phenotypic flexibility and the evolution of organismal design. Trends Ecol Evol 18(5):228–233
Piersma T, van Gils JA (2010) The Flexible phenotype: a body-centred integration of ecology, physiology, and behaviour. Oxford University Press, Oxford
Pigliucci M (2001) Phenotypic plasticity: beyond nature and nurture. Johns Hopkins University Press, Baltimore
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Richards CL, Bossdorf O, Muth NZ, Gurevitch J, Pigliucci M (2006) Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecol Lett 9(8):981–993
Sanford E, Kelly MW (2011) Local adaptation in marine invertebrates. Ann Rev Mar Sci 3(1):509–535
Scheltema RS (1978) On the relationship between dispersal of pelagic veliger larvae and the evolution of marine prosobranch gastropods. In: Battaglia B (ed) Marine organisms, Genetics, Ecology, and Evolution. pp 303–322
Serafini L, Hann JB, Kultz D, Tomanek L (2011) The proteomic response of sea squirts (genus Ciona) to acute heat stress: a global perspective on the thermal stability of proteins. Comp Biochem Physiol D 6(3):322–334
Sotka EE (2012) Natural selection, larval dispersal, and the geography of phenotype in the sea. Integr Comp Biol 52(4):538–545
Strathmann RR (1986) What controls the type of larval development? Summary statement for the evolution session. Bull Mar Sci 39(2):616–622
Svane I, Havenhand JN (1993) Spawning and dispersal in Ciona intestinalis (L.). Mar Ecol 14(1):53–66
Svane I, Havenhand JN, Jørgensen AJ (1987) Effects of tissue extract of adults on metamorphosis in Ascidia mentula O.F. Müller and Ascidiella scabra (O.F. Müller). J Exp Mar Biol Ecol 110(2):171–181
Therriault TW, Herborg L-M (2008) Predicting the potential distribution of the vase tunicate Ciona intestinalis in Canadian waters: informing a risk assessment. ICES J Mar Sci 65(5):788–794
Via S, Lande R (1985) Genotype-environment interaction and the evolution of phenotypic plasticity. Evolution 39(3):505–522
West-Eberhard MJ (1986) Animal behaviour: experimental behavioural ecology and sociobiology. Science 231(4733):64–65
West-Eberhard MJ (2005) Developmental plasticity and the origin of species differences. Proc Natl Acad Sci USA 102(Suppl 1):6543–6549
Whitman DW, Agrawal AA (2009) What is phenotypic plasticity and why is it important? In: Whitman DW, Ananthakrishnan TN (eds) Phenotypic plasticity of insects: mechanisms and consequences. Science Publishers, Enfield
Zerebecki RA, Sorte CJB (2011) Temperature tolerance and stress proteins as mechanisms of invasive species success. PLoS One 6(4):e14806
Zhan A, Macisaac HJ, Cristescu ME (2010) Invasion genetics of the Ciona intestinalis species complex: from regional endemism to global homogeneity. Mol Ecol 19(21):4678–4694
Zhan A, Darling JA, Bock DG, Lacoursiere-Roussel A, Macisaac HJ, Cristescu ME (2012) Complex genetic patterns in closely related colonizing invasive species. Ecol Evol 2(7):1331–1346
Acknowledgments
We’d like to thank Gerry Quinn (Deakin University) for statistical expertise, and Daniel Simonsson and Martin Ogemark for technical assistance in the laboratory. This study was supported by the project ECOSUPPORT (Advanced modeling tool for scenarios of the Baltic Sea ECOsystem to SUPPORT decision making) under the EU 7th Framework Programme (FP/2007-2013) BONUS programme, and was partly undertaken within the Linnaeus Centre for Marine Evolutionary Biology (http://www.cemeb.science.gu.se/), supported by a Linnaeus-grant from the Swedish Research Councils VR and Formas.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10682_2013_9687_MOESM1_ESM.eps
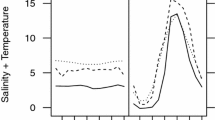
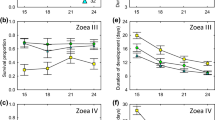
Figure 4 Salinity tolerance of metamorphosis in Ciona larvae from Gullmarsfjorden Shallow (a), Väderöarna Shallow (b), Gullmarsfjorden Deep (c) and Väderöarna Deep (d) acclimated to native and non-native salinities (25 and 31 PSU). Data points are for individual replicates showing the two sites Gullmarsfjorden and Väderöarna separately, where curves are LOESS smooths (span =0,5) and grey shading indicates 95‰ CI of the curves. (EPS 1472 kb)
Rights and permissions
About this article
Cite this article
Renborg, E., Johannesson, K. & Havenhand, J. Variable salinity tolerance in ascidian larvae is primarily a plastic response to the parental environment. Evol Ecol 28, 561–572 (2014). https://doi.org/10.1007/s10682-013-9687-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-013-9687-2