Abstract
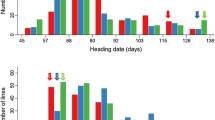
The main objective of this study was to dissect the genetic basis of rice flowering regulated by temperature. We used two indica varieties Zhenshan 97 and Zhongzao 18, and 168 recombinant inbred lines (RILs) derived from them to estimate temperature effects on heading date (HD). Tests under four different photoperiod and temperature conditions in growth chambers showed that HD of both parents was accelerated at high temperatures, indicating that both parents were strongly thermo-sensitive. The averaged effective cumulative temperatures (ECTs), 1,120 and 1,263 °C necessary for Zhenshan 97 and Zhongzao 18 respectively, could be the prerequisite in a given condition before heading. QTL analysis was conducted with the RILs grown in both long day and short day conditions in 2 years. A total of 4 ECT QTLs corresponding to 4 HD QTLs were mapped. The major ECT QTL, qEHD10, was repeatedly identified on chromosome 10, which could explain 42.2–57.0 % of ECT variation and 39.8–59.4 % of HD variation in four environments. Overall these novel findings would improve the knowledge of temperature on rice flowering.



Similar content being viewed by others
References
Balasubramanian S, Sureshkumar S, Lempe J, Weigel D (2006) Potent induction of Arabidopsis thaliana flowering by elevated growth temperature. PLoS Genet 2(7):e106. doi:10.1371/journal.pgen.0020106
Barth C, De Tullio M, Conklin PL (2006) The role of ascorbic acid in the control of flowering time and the onset of senescence. J Exp Bot 57(8):1657–1665. doi:10.1093/jxb/erj198
Blazquez MA, Ahn JH, Weigel D (2003) A thermosensory pathway controlling flowering time in Arabidopsis thaliana. Nat Genet 33(2):168–171. doi:10.1038/ng1085
Chang TT, Li CC, Vergara BS (1969) Component analysis of duration from seeding to heading in rice by the basic vegetative phase and the photoperiod-sensitive phase. Euphytica 18:79–91
Doi K, Izawa T, Fuse T, Yamanouchi U, Kubo T, Shimatani Z, Yano M, Yoshimura A (2004) Ehd1, a B-type response regulator in rice, confers short-day promotion of flowering and controls FT-like gene expression independently of Hd1. Genes Dev 18(8):926–936. doi:10.1101/gad.1189604
Ellis RH, Qi A, Summerfield RJ, Roberts EH (1993) Rates of leaf appearance and panicle development in rice (Oryza sativa L.): a comparison at three temperatures. Agric For Meteorol 66:129–138
Gao LZ, Jin ZQ, Li L (1987) Photo-thermal models of rice growth duration for various varietal types in China. Agric For Meteorol 39:205–213
Garner WW, Allard HA (1920) Effect of the relative length of day and night and other factors of the environment on growth and reproduction in plants. J Agric Res 18:553–606
Guo LB, Zhu LH, Xu YB, Zeng DL, Wu P, Qian Q (2004) QTL analysis of seed dormancy in rice (Oryza sativa L.). Euphytica 140:155–162
Hayama R, Yokoi S, Tamaki S, Yano M, Shimamoto K (2003) Adaptation of photoperiodic control pathways produces short-day flowering in rice. Nature 422(6933):719–722. doi:10.1038/nature01549
International Rice Genome Sequencing P (2005) The map-based sequence of the rice genome. Nature 436(7052):793–800. doi:10.1038/nature03895
Izawa T (2007) Day length measurements by rice plants in photoperiodic short day flowering. Int Rev Cytol 256:191–222
Kim SL, Lee S, Kim HJ, Nam HG, An G (2007) OsMADS51 is a short-day flowering promoter that functions upstream of Ehd1, OsMADS14, and Hd3a. Plant Physiol 145(4):1484–1494. doi:10.1104/pp.107.103291
Kobayashi Y, Kaya H, Goto K, Iwabuchi M, Araki T (1999) A pair of related genes with antagonistic roles in mediating flowering signals. Science 286(5446):1960–1962
Kojima S, Takahashi Y, Kobayashi Y, Monna L, Sasaki T, Araki T, Yano M (2002) Hd3a, a rice ortholog of the Arabidopsis FT gene, promotes transition to flowering downstream of Hd1 under short-day conditions. Plant Cell Physiol 43(10):1096–1105
Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, Newberg LA (1987) MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics 1(2):174–181
Lee I, Bleecker A, Amasino R (1993) Analysis of naturally occurring late flowering in Arabidopsis thaliana. Mol Genet Genomics 237(1–2):171–176
Lee JH, Yoo SJ, Park SH, Hwang I, Lee JS, Ahn JH (2007) Role of SVP in the control of flowering time by ambient temperature in Arabidopsis. Genes Dev 21(4):397–402. doi:10.1101/gad.1518407
Leon AJ, Lee M, Andrade FH (2001) Quantitative trait loci for growing degree days to flowering and photoperiod response in sunflower (Helianthus annuus L.). Theor Appl Genet 102:497–503
Levy YY, Mesnage S, Mylne JS, Gendall AR, Dean C (2002) Multiple roles of Arabidopsis VRN1 in vernalization and flowering time control. Science 297(5579):243–246. doi:10.1126/science.1072147
Lin HX, Yamamoto T, Sasaki T, Yano M (2002) Identification and characterization of a quantitative trait locus, Hd9, controlling heading date in rice. Breed Sci 52:35–41
Lincoln S, Daly M, Lander E (1993) Mapping genes controlling quantitative traits with MAPMAKER/QTL 1.1: a tutorial and reference manual, 2nd edn. Whitehead Institute, Cambridge
Luan W, Chen H, Fu Y, Si H, Peng W, Song S, Liu W, Hu G, Sun Z, Xie D, Sun C (2009) The effect of the crosstalk between photoperiod and temperature on the heading-date in rice. PLoS One 4(6):e5891. doi:10.1371/journal.pone.0005891
Matsubara K, Yamanouchi U, Wang ZX, Minobe Y, Izawa T, Yano M (2008) Ehd2, a rice ortholog of the maize INDETERMINATE1 gene, promotes flowering by up-regulating Ehd1. Plant Physiol 148(3):1425–1435. doi:10.1104/pp.108.125542
Matsubara K, Hori K, Ogiso-Tanaka E, Yano M (2014) Cloning of quantitative trait genes from rice reveals conservation and divergence of photoperiod flowering pathways in Arabidopsis and rice. Front Plant Sci 5:193. doi:10.3389/fpls.2014.00193
McCouch SR, Teytelman L, Xu Y, Lobos KB, Clare K, Walton M, Fu B, Maghirang R, Li Z, Xing Y, Zhang Q, Kono I, Yano M, Fjellstrom R, DeClerck G, Schneider D, Cartinhour S, Ware D, Stein L (2002) Development and mapping of 2,240 new SSR markers for rice (Oryza sativa L.). DNA Res 9:199–207
McMaster GS, White JW, Hunt LA, Jamieson PD, Dhillon SS, Ortiz-Monasterio JI (2008) Simulating the influence of vernalization, photoperiod and optimum temperature on wheat developmental rates. Ann Bot 102(4):561–569. doi:10.1093/aob/mcn115
Michaels SD, Amasino RM (1999) FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 11(5):949–956
Mouradov A, Cremer F, Coupland G (2002) Control of flowering time: interacting pathways as a basis for diversity. Plant Cell 14:S111–S130. doi:10.1105/tpc.001362
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8(19):4321–4325
Nakagawa H, Yamagishi J, Miyamoto N, Motoyama M, Yano M, Nemoto K (2005) Flowering response of rice to photoperiod and temperature: a QTL analysis using a phenological model. Theor Appl Genet 110(4):778–786. doi:10.1007/s00122-004-1905-4
Park SJ, Kim SL, Lee S, Je BI, Piao HL, Park SH, Kim CM, Ryu CH, Park SH, Xuan YH, Colasanti J, An G, Han CD (2008) Rice indeterminate 1 (OsId1) is necessary for the expression of Ehd1 (Early heading date 1) regardless of photoperiod. Plant J 56(6):1018–1029. doi:10.1111/j.1365-313X.2008.03667.x
Poethig RS (2003) Phase change and the regulation of developmental timing in plants. Science 301(5631):334–336. doi:10.1126/science.1085328
Riechmann JL, Heard J, Martin G, Reuber L, Jiang C-Z, Keddie J, Adam L, Pineda O, Ratcliffe OJ, Samaha RR, Creelman R, Pilgrim M, Broun P, Zhang JZ, Ghandehari D, Sherman BK, Yu G-L (2000) Arabidopsis transcription factors: genome-wide comparative analysis among eukaryotes. Science 290:2105
Robson F, Costa MM, Hepworth SR, Vizir I, Pineiro M, Reeves PH, Putterill J, Coupland G (2001) Functional importance of conserved domains in the flowering-time gene CONSTANS demonstrated by analysis of mutant alleles and transgenic plants. Plant J 28(6):619–631
Saito H, Yuan Q, Okumoto Y, Doi K, Yoshimura A, Inoue H, Teraishi M, Tsukiyama T, Tanisaka T (2009) Multiple alleles at Early flowering 1 locus making variation in the basic vegetative growth period in rice (Oryza sativa L.). Theor Appl Genet. doi:10.1007/s00122-009-1040-3
Sawa M, Nusinow DA, Kay SA, Imaizumi T (2007) FKF1 and GIGANTEA complex formation is required for day-length measurement in Arabidopsis. Science 318(5848):261–265. doi:10.1126/science.1146994
Shrestha R, Gomez-Ariza J, Brambilla V, Fornara F (2014) Molecular control of seasonal flowering in rice, Arabidopsis and temperate cereals. Ann Bot. doi:10.1093/aob/mcu032
Summerfield RJ, Collinson ST, Ellis RH, Roberts EH, Penning de Vries FWT (1992) Photothermal responses of flowering in rice (Oryza sativa). Ann Bot 69:101–112
Sung S, Amasino RM (2004) Vernalization in Arabidopsis thaliana is mediated by the PHD finger protein VIN3. Nature 427(6970):159–164. doi:10.1038/nature02195
Temnykh S, Park WD, Ayres N, Cartihour S, Hauck N, Lipovich L, Cho YG, Ishii T, McCouch SR (2000) Mapping and genome organization of microsatellite sequences in rice (Oryza sativa L.). Theor Appl Genet 100:697–712
Temnykh S, DeClerck G, Lukashova A, Lipovich L, Cartinhour S, McCouch S (2001) Computational and experimental analysis of microsatellites in rice (Oryza sativa L.): frequency, length variation, transposon associations, and genetic marker potential. Genome Res 11(8):1441–1452. doi:10.1101/gr.184001
Turck F, Fornara F, Coupland G (2008) Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annu Rev Plant Biol 59:573–594. doi:10.1146/annurev.arplant.59.032607.092755
Vergara BS, Chang TT (1985) The flowering response of the rice plant to photoperiod, 4th edn. The International Rice Research Institute, Manila
Warner RM, Erwin JE (2005) Naturally occurring variation in high temperature induced floral bud abortion across Arabidopsis thaliana accessions. Plant Cell Environ 28:1255–1266
Weiss A, Hays CJ (2005) Calculating daily mean air temperatures by different methods: implications from a non-linear algorithm. Agric For Meteorol 128(1–2):57–65. doi:10.1016/j.agrformet.2004.08.008
Wu KS, Tanksley SD (1993) Abundance, polymorphism and genetic mapping of microsatellites in rice. Mol Gen Genet 241(1–2):225–235
Wu C, You C, Li C, Long T, Chen G, Byrne ME, Zhang Q (2008) RID1, encoding a Cys2/His2-type zinc finger transcription factor, acts as a master switch from vegetative to floral development in rice. Proc Natl Acad Sci USA 105(35):12915–12920. doi:10.1073/pnas.0806019105
Xie LW (2002) Effect of applying effective accumulative temperature difference method on flowering synchronization in seed production of new hybrids. Hybrid Rice 17:29–30
Xue DW, Fang MD, Qian Q (2004) Application of effective cumulative temperature in rice. China Rice 4:47–48
Xue W, Xing Y, Weng X, Zhao Y, Tang W, Wang L, Zhou H, Yu S, Xu C, Li X, Zhang Q (2008) Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat Genet 40(6):761–767. doi:10.1038/ng.143
Yan WH, Wang P, Chen HX, Zhou HJ, Li QP, Wang CR, Ding ZH, Zhang YS, Yu SB, Xing YZ, Zhang QF (2011) A major QTL, Ghd8, plays pleiotropic roles in regulating grain productivity, plant height, and heading date in rice. Mol Plant 4(2):319–330. doi:10.1093/mp/ssq070
Yan W, Liu H, Zhou X, Li Q, Zhang J, Lu L, Liu T, Liu H, Zhang C, Zhang Z, Shen G, Yao W, Chen H, Yu S, Xie W, Xing Y (2013) Natural variation in Ghd7.1 plays an important role in grain yield and adaptation in rice. Cell. doi:10.1038/cr.2013.43
Yano M, Katayose Y, Ashikari M, Yamanouchi U, Monna L, Fuse T, Baba T, Yamamoto K, Umehara Y, Nagamura Y, Sasaki T (2000) Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS. Plant Cell 12(12):2473–2484
Yin X, Kropff MJ, McLaren G, Visperas RM (1995) A nonlinear model for crop development as a function of temperature. Agric For Meteorol 77:1–16
Acknowledgments
This work was supported by Grants from the National Natural Science Foundation of China (91335201), National Key Program on Basic Research Projects (2010CB125901) and the Bill & Melinda Gates Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mallikarjuna Rao Kovi and Yong Hu have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kovi, M.R., Hu, Y., Bai, X. et al. QTL mapping for thermo-sensitive heading date in rice. Euphytica 205, 51–62 (2015). https://doi.org/10.1007/s10681-015-1383-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-015-1383-6