Abstract
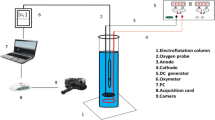
The performance of electroflotation (EF) is strongly influenced by the size of O2 and H2 bubbles. Therefore, in this study, the bubble sizes are measured in a lab-scale EF cell using a high-speed camera. The mean bubble size is found to vary in the range of 32.7–68.6 μm under different operating conditions. This study shows that the electrode material, current density, water pH, ionic strength, and frother (Tennafroth 250) concentration are important factors in controlling the bubble size. Furthermore, four mathematical distributions (normal, log-normal, Weibull, and gamma distributions) are fitted to the experimental data, among which the log-normal distribution is found to be the best fit based on the lower Anderson-Darling (AD) value.

















Similar content being viewed by others
References
Ahmed, N., & Jameson, G. J. (1985). The effect of bubble size on the rate of flotation of fine particles. International Journal of Mineral Processing, 14, 195–215.
Al-Hayes, R. A. M., & Winterton, R. H. S. (1981). Bubble diameter on detachment in flowing liquids. International Journal of Heat and Mass Transfer, 24, 213–331.
Alam, R., & Shang, J. Q. (2016). Electrochemical model of electro-flotation. Journal of Water Process Engineering, 12, 78–88.
Alam, R., & Shang, J. Q. (2017). Removal of bitumen from mature oil sands tailings slurries by electro-flotation. Journal of Water Process Engineering, 15, 116–123.
Alam, R., Shang, J. Q., & Cheng, X. (2011). Optimization of digestion parameters for analyzing the total sulphur of mine tailings by inductive coupled plasma optical emission spectrometry. Environmental Monitoring and Assessment (Springer), 184(5)), 3373–3387.
Alam, R., Shang, J. Q., & Islam, S. (2017). Electrophoresis and its applications in oil sand tailings management. International Journal of Mineral Processing, 161, 41–49.
Bande, R. M., Prasad, B., Mishra, I. M., & Wasewar, K. L. (2008). Oil field effluent water treatment for safe disposal by electro-flotation. Chemical Engineering Journal, 137, 503–509.
Beer, H. B. 1972. U.S. Patent 3,632,498.
Bennet, A. J. R., Chapmen, W. R., Dell, C. C. 1958. Studies in the froth flotation of coal. Third International Coal Preparation Conngress, Brussels-Leige.
Biswal, S. K., Reddy, P. S. R., & Bhaumik, S. K. (2009). Bubble size distribution in a flotation column. The Canadian Journal of Chemical Engineering, 72(1), 148–152. doi:10.1002/cjce.5450720123.
Brandon, N. P., & Kelsall, G. H. (1985). Interfacial electrical properties of electrogenerated bubbles. Journal of Applied Electrochemistry, 15, 485–493.
Burns, S. E., Yiacoumi, S., & Tsouris, C. (1997). Microbubble generation for environmental and industrial separations. Separation and Purification Technology, 11, 221–232.
Chen, X., Chen, G. 2010. Electro-flotation. In C. Comninellis & G. Chen (Eds.), Electrochemistry for the environment, Springer Science+Business Media, LLC. 263–77.
Cho, Y. S., & Laskowski, J. S. (2002). Effect of flotation frothers on bubble size and foam stability. International Journal of Mineral Processing, 64, 69–80.
Coleman, R. 2009. Outotec, more out of ore. Outotec Australia’s quarterly e-newsletter, issue 25.
Cruz, S. G., Dutra, A. J. B., & Monte, M. B. M. (2016). The influence of some parameters on bubble average diameter in an electroflotation cell by laser diffraction method. Journal of Environmental Chemical Engineering, 4, 3681–3687.
Dai, J., Xie, G., Liu, S., & Wang, X. (2007). Analysis of influencing factors of flotation bubble size. Coal Preparation Technology, 3, 7–10.
Fatima, M., & Fortes, M. A. (1988). Grain size distribution: the log-normal and the gamma distribution function. Scripta Metallurgica, 22, 35–40.
Fukui, Y., & Yuu, S. (1985). Removal of colliodal particles in electro-flotation. AICHE Journal, 31(2), 201–208.
Glembotsky, V. A., Mamakov, A. A., & Sorokina, V. N. (1973). Electroannaya Obrabotka Materialov, 5, 66.
Gorain, B. K., Franzidis, J.-P., & Manlapig, E. V. (1995a). Studies on impeller type, impeller speed and air flow rate in an industrial scale flotation cell—part 1: effect on bubble size distribution. Minerals Engineering, 8(6), 615–635.
Gorain, B. K., Franzidis, J.-P., & Manlapig, E. V. (1995b). Studies on impeller type, impeller speed and air flow rate in an industrial scale flotation cell part 2: effect on gas holdup. Minerals Engineering, 8(12), 1557–1570.
Gorain, B. K., Franzidis, J.-P., & Manlapig, E. V. (1997). Studies on impeller type, impeller speed and air flow rate in an industrial scale flotation cell. Part 4: effect of bubble surface area flux on flotation performance. Minerals Engineering, 10(4), 367–379.
Grau, R. A., & Heiskanen, K. (2005). Bubble size distribution in laboratory scale flotation cells. Minerals Engineering, 18, 1164–1172.
Grau, R. A., & Laskowski, J. S. (2006). Role of frothers in bubble generation and coalescence in a mechanical flotation cell. The Canadian Journal of Chemical Engineering, 84, 170–182.
Grau, R. A., Laskowski, J. S., & Heiskanen, K. (2005). Effect of frothers on bubble size. International Journal of Mineral Processing, 76, 225–233.
Heiskanen, K. (2000). On the relationship between flotation rate and bubble surface area flux. Minerals Engineering, 13(2), 141–149.
Ibl, N., & Venczel, I. (1970). Metalloberflache, 34, 365.
Jiménez, C., Talavera, B., Sáez, C., Canizares, P., & Rodrigo, M. A. (2010). Study of the production of hydrogen bubbles at low current densities for electro-flotation processes. Journal of Chemical Technology and Biotechnology, 85, 1368–1373.
Ketkar, D. R., Mallikarjunan, R., & Venkatachalam, S. (1991). Electro-flotation ofquartz fines. International Journal of Mineral Processing, 31, 127–138.
Khosla, N. K., Venkatachalam, S., & Somasundaran, P. (1991). Pulsed electrogeneration of bubbles for electro-flotation. Journal of Applied Electrochemistry, 21, 986–990.
Landolt, D., Acosta, R., Muller, R. H., & Tobais, C. W. (1970). An optical study of cathodic hydrogen evolution in high rate electrolysis. Journal of the Electrochemical Society, 117(6), 839–845.
Lee, S. M. (1969). Effect of equivalent bubble sizes on the flotability of single bubble forth flotation. Journal of AIChE, 7, 202–213.
Liuyi, R., Zhang, Y., Qin, W., Bao, S., Wang, P., & Yang, C. (2014). Investigation of condition-induced bubble size and distribution in electro-flotation using a high-speed camera. International Journal of Mining Science and Technology, 24, 7–12.
Lumanauw, D. 2000. Hydrogen bubble characterization in alkaline water electrolysis, thesis of Master of Applied Science, Department of Metallurgy and Materials Science, University of Toronto.
Mÿmicci, G., & Nicoderno, L. (1967). Chemical Engineering Science, 27, 1257.
Oktepe, G. F. (2002). Effect of pH on pulp potential and sulphide mineral flotation. Turkish Journal of Engineering and Environmental Science, 26, 309–318.
Pacek, A., Man, C., & Nienow, A. (1998). On the Sauter mean diameter and size distributions in turbulent liquid/liquid dispersions in a stirred vessel. Chemical Engineering Science, 53(11), 2005–2011.
Raju, G. B., & Khangaonkar. (1984). Electro-flotation—a critical review. Transactions of the Indian Institute of Metals, 37(1), 59–66.
Sarkar, M. S. K. A., Evans, G. M., & Donne, S. W. (2010a). Bubble size measurement in electro-flotation. Minerals Engineering, 23, 1058–1065.
Sarkar, M. S. K. A., Donne, S. W., & Evans, G. M. (2010b). Hydrogen bubble flotation of silica. Advanced Powder Technology, 21, 412–418.
Sides, P. J. (1986). Modern aspects of electrochemistry. In R. E. White, J. O. N. Bokris, & B. E. Conway (Eds.), Phenomenon and effects of electrolytic gas evolution (pp. 303–354). New York: Plenum.
Silva, EL and Lisboa, P. 2007. Analysis of the characteristic features of the density functions for gamma, Weibull and log-normal distributions through RBF network pruning with QLP Proceedings of the 6th WSEAS Int. Conf. on Artificial Intelligence, Knowledge Engineering and Data Bases, Corfu Island, Greece, February 16–19, 2007
Stephens, M. A. (1974). EDF statistics for goodness of fit and some comparisons. Journal of the American Statistical Association, 69, 730–737.
Tavlarides, L., & Stamatoudis, M. (1981). The analysis of interphase reactions and mass transfer in liquid–liquid dispersions. Advances in Chemical Engineering, 11, 199–273.Tijms, H.C. (2003). A first course in stochastic models. Ámsterdam :Vrije Universiteit
Vogt, H. (1984). The rate of gas evolution of electrodes—I. An estimate of the efficiency of gas evolution from the supersaturation of electrolyte adjacent to a gas-evolving electrode. Electrochimica Acta, 29(2), 167–173.
Vogt, H. (1989). The problem of the departure diameter of bubbles at gas-evolving electrodes. Electrochemica Acta, 34(10), 1429–1432.Vogt, H. (1983). In Comprehensive treatise of electrochemistry (edited by E. Yeager, J. O’M. Bockris, B. E. Conway and S. Sarangapani), Plenum Press, New York, Vol. 6, 445.
Wei, S., Liang, M., Yuehua, H., Yanhong, D., & Gang, Z. 2011. Hydrogen bubble flotation of fine minerals containing calcium. Mining Science and Technology (China), 21(2011), 591–597.
Yoon, R.-H. (2000). International Journal of Mineral Processing, 58, 129–143.
Zhang, L., Li, T., Ying, W.-y., & Fang, D.-y. (2008). Rising and descending bubble size distributions in gas–liquid and gas–liquid–solid slurry bubble column reactor. Chemical Engineering Research and Design, 86, 1143–1154.
Ziemenski, S. A., & Whittemore, R. C. (1971). Chemical Engineering Science, 26, 509–520.
Acknowledgements
The authors would like to thank Mr. Puneet Verma for his help during the research. The authors are also thankful to the Associate Editor of EMAS and the anonymous reviewers for their valuable suggestions to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alam, R., Shang, J.Q. & Khan, A.H. Bubble size distribution in a laboratory-scale electroflotation study. Environ Monit Assess 189, 193 (2017). https://doi.org/10.1007/s10661-017-5888-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-017-5888-4