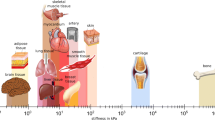
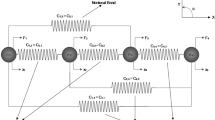
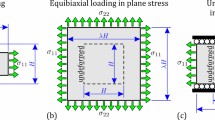
Abstract
This article illustrates our approach for modeling the solid matrix of biological tissues using reactive constrained mixtures. Several examples are presented to highlight the potential benefits of this approach, showing that seemingly disparate fields of mechanics and chemical kinetics are actually closely interrelated and may be elegantly expressed in a unified framework. Thus, constrained mixture models recover classical theories for fibrous materials with bundles oriented in different directions or having different reference configurations, that produce characteristic fiber recruitment patterns under loading. Reactions that exchange mass among various constituents of a mixture may be used to describe tissue growth and remodeling, which may also alter the material’s anisotropy. Similarly, reactions that describe the breaking and reforming of bonds may be used to model free energy dissipation in a viscoelastic material. Therefore, this framework is particularly well suited for modeling biological tissues.
















Similar content being viewed by others
Notes
For an infinitesimal strain analysis we may assume that the material and spatial configurations are nearly identical, \(x\approx X^{s}\).
Chemistry textbooks typically adopt temperature and pressure as state variables, so that the Gibbs energy emerges as the natural scalar potential for examining the thermodynamics of reactions. In contrast, our treatment employs temperature and strain (thus, volume) as state variables, so that the Helmholtz free energy emerges as the natural choice of scalar potential.
An explicit dependence of \(E\) on \(\rho_{r}^{\alpha }\) may also be adopted, e.g., assuming that each bundle has the same density \(\rho_{r}^{\alpha }=\rho_{0}/n\), where \(\rho_{0}\) is the mixture density; then, letting \(E ( \rho_{r}^{ \alpha } ) = ( \rho_{r}^{\alpha }/\rho_{0} ) E_{0}\) would reproduce the relation adopted in (3.14b).
References
Armstrong, C.G.: An analysis of the stresses in a thin layer of articular cartilage in a synovial joint. Eng. Med. 15(2), 55–61 (1986)
Armstrong, C.G., Lai, W.M., Mow, V.C.: An analysis of the unconfined compression of articular cartilage. J. Biomech. Eng. 106(2), 165–173 (1984)
Aspden, R.M., Hukins, D.W.: Collagen organization in articular cartilage, determined by X-ray diffraction, and its relationship to tissue function. Proc. R. Soc. Lond. B, Biol. Sci. 212(1188), 299–304 (1981)
Ateshian, G.A.: On the theory of reactive mixtures for modeling biological growth. Biomech. Model. Mechanobiol. 6(6), 423–445 (2007)
Ateshian, G.A.: Viscoelasticity using reactive constrained solid mixtures. J. Biomech. 48(6), 941–947 (2015)
Ateshian, G.A., Ellis, B.J., Weiss, J.A.: Equivalence between short-time biphasic and incompressible elastic material responses. J. Biomech. Eng. 129(3), 405–412 (2007)
Ateshian, G.A., Morrison, B. III, Holmes, J.W., Hung, C.T.: Mechanics of cell growth. Mech. Res. Commun. 42, 118–125 (2012)
Ateshian, G.A., Rajan, V., Chahine, N.O., Canal, C.E., Hung, C.T.: Modeling the matrix of articular cartilage using a continuous fiber angular distribution predicts many observed phenomena. J. Biomech. Eng. 131(6), 061003 (2009)
Ateshian, G.A., Ricken, T.: Multigenerational interstitial growth of biological tissues. Biomech. Model. Mechanobiol. 9(6), 689–702 (2010)
Azeloglu, E.U., Albro, M.B., Thimmappa, V.A., Ateshian, G.A., Costa, K.D.: Heterogeneous transmural proteoglycan distribution provides a mechanism for regulating residual stresses in the aorta. Am. J. Physiol., Heart Circ. Physiol. 294(3), H1197–H1205 (2008)
Bachrach, N.M., Mow, V.C., Guilak, F.: Incompressibility of the solid matrix of articular cartilage under high hydrostatic pressures. J. Biomech. 31(5), 445–451 (1998)
Bedford, A., Drumheller, D.S.: Theories of immiscible and structured mixtures. Int. J. Eng. Sci. 21(8), 863–960 (1983)
Bowen, R.M.: Thermochemistry of reacting materials. J. Chem. Phys. 49(4), 1625–1637 (1968)
Bryant, M.R., McDonnell, P.J.: A triphasic analysis of corneal swelling and hydration control. J. Biomech. Eng. 120(3), 370–381 (1998)
Carter, D.R., Hayes, W.C.: Bone compressive strength: the influence of density and strain rate. Science 194(4270), 1174–1176 (1976)
Chahine, N.O., Wang, C.C.-B., Hung, C.T., Ateshian, G.A.: Anisotropic strain-dependent material properties of bovine articular cartilage in the transitional range from tension to compression. J. Biomech. 37(8), 1251–1261 (2004)
Coleman, B.D., Gurtin, M.E.: Thermodynamics with internal state variables. J. Chem. Phys. 47(2), 597–613 (1967)
Cowin, S., Hegedus, D.: Bone remodeling. I: Theory of adaptive elasticity. J. Elast. 6(3), 313–326 (1976)
Cyron, C.J., Aydin, R.C., Humphrey, J.D.: A homogenized constrained mixture (and mechanical analog) model for growth and remodeling of soft tissue. Biomech. Model. Mechanobiol. 15(6), 1389–1403 (2016)
Eberhardt, A.W., Keer, L.M., Lewis, J.L., Vithoontien, V.: An analytical model of joint contact. J. Biomech. Eng. 112(4), 407–413 (1990)
Eringen, A.C., Ingram, J.D.: A continuum theory of chemically reacting media—I. Int. J. Eng. Sci. 3(2), 197–212 (1965)
Fyhrie, D.P., Carter, D.R.: A unifying principle relating stress to trabecular bone morphology. J. Orthop. Res. 4(3), 304–317 (1986)
Gasser, T.C., Ogden, R.W., Holzapfel, G.A.: Hyperelastic modelling of arterial layers with distributed collagen fibre orientations. J. R. Soc. Interface 3(6), 15–35 (2006)
Gleason, R.L., Humphrey, J.D.: Effects of a sustained extension on arterial growth and remodeling: a theoretical study. J. Biomech. 38(6), 1255–1261 (2005)
Gleason, R.L., Taber, L.A., Humphrey, J.D.: A 2-d model of flow-induced alterations in the geometry, structure, and properties of carotid arteries. J. Biomech. Eng. 126(3), 371–381 (2004)
Green, M., Tobolsky, A.: A new approach to the theory of relaxing polymeric media. J. Chem. Phys. 14(2), 80–92 (1946)
Hill, M.R., Duan, X., Gibson, G.A., Watkins, S., Robertson, A.M.: A theoretical and non-destructive experimental approach for direct inclusion of measured collagen orientation and recruitment into mechanical models of the artery wall. J. Biomech. 45(5), 762–771 (2012)
Hori, R.Y., Mockros, L.F.: Indentation tests of human articular cartilage. J. Biomech. 9(4), 259–268 (1976)
Huiskes, R., Weinans, H., Grootenboer, H.J., Dalstra, M., Fudala, B., Slooff, T.J.: Adaptive bone-remodeling theory applied to prosthetic-design analysis. J. Biomech. 20(11–12), 1135–1150 (1987)
Humphrey, J., Rajagopal, K.: A constrained mixture model for growth and remodeling of soft tissues. Math. Models Methods Appl. Sci. 12(03), 407–430 (2002)
Humphrey, J.D., Rajagopal, K.R.: A constrained mixture model for arterial adaptations to a sustained step change in blood flow. Biomech. Model. Mechanobiol. 2(2), 109–126 (2003)
Hurschler, C., Loitz-Ramage, B., Vanderby, R. Jr.: A structurally based stress-stretch relationship for tendon and ligament. J. Biomech. Eng. 119(4), 392–399 (1997)
Karsaj, I., Humphrey, J.D.: A mathematical model of evolving mechanical properties of intraluminal thrombus. Biorheology 46(6), 509–527 (2009)
Kenyon, D.E.: The theory of an incompressible solid-fluid mixture. Arch. Ration. Mech. Anal. 62(2), 131–147 (1976)
Lake, S.P., Miller, K.S., Elliott, D.M., Soslowsky, L.J.: Effect of fiber distribution and realignment on the nonlinear and inhomogeneous mechanical properties of human supraspinatus tendon under longitudinal tensile loading. J. Orthop. Res. 27(12), 1596–1602 (2009)
Lanir, Y.: A structural theory for the homogeneous biaxial stress-strain relationships in flat collagenous tissues. J. Biomech. 12(6), 423–436 (1979)
Lanir, Y.: Constitutive equations for fibrous connective tissues. J. Biomech. 16(1), 1–12 (1983)
Mak, A.F., Lai, W.M., Mow, V.C.: Biphasic indentation of articular cartilage—I: Theoretical analysis. J. Biomech. 20(7), 703–714 (1987)
Mansour, J.M., Mow, V.C.: The permeability of articular cartilage under compressive strain and at high pressures. J. Bone Jt. Surg., Am. Vol. 58(4), 509–516 (1976)
Maroudas, A., Bannon, C.: Measurement of swelling pressure in cartilage and comparison with the osmotic pressure of constituent proteoglycans. Biorheology 18(3–6), 619–632 (1981)
Mow, V., Kuei, S., Lai, W., Armstrong, C.: Biphasic creep and stress relaxation of articular cartilage in compression: theory and experiments. J. Biomech. Eng. 102(1), 73–84 (1980)
Nims, R.J., Durney, K.M., Cigan, A.D., Dusséaux, A., Hung, C.T., Ateshian, G.A.: Continuum theory of fibrous tissue damage mechanics using bond kinetics: application to cartilage tissue engineering. Interface Focus 6(1), 20150063 (2016)
Oloyede, A., Broom, N.D.: Is classical consolidation theory applicable to articular cartilage deformation? Clin. Biomech. 6(4), 206–212 (1991)
Park, S., Krishnan, R., Nicoll, S.B., Ateshian, G.A.: Cartilage interstitial fluid load support in unconfined compression. J. Biomech. 36(12), 1785–1796 (2003)
Pierce, D.M., Ricken, T., Holzapfel, G.A.: Modeling sample/patient-specific structural and diffusional responses of cartilage using dt-mri. Int. J. Numer. Methods Biomed. Eng. 29(8), 807–821 (2013)
Prud’homme, R.: Flows of Reactive Fluids. Fluid Mechanics and Its Applications, vol. 94. Springer, New York (2010)
Rachev, A., Gleason, R.L. Jr.: Theoretical study on the effects of pressure-induced remodeling on geometry and mechanical non-homogeneity of conduit arteries. Biomech. Model. Mechanobiol. 10(1), 79–93 (2011)
Sacks, M.S.: Incorporation of experimentally-derived fiber orientation into a structural constitutive model for planar collagenous tissues. J. Biomech. Eng. 125(2), 280–287 (2003)
Satha, G., Lindström, S.B., Klarbring, A.: A goal function approach to remodeling of arteries uncovers mechanisms for growth instability. Biomech. Model. Mechanobiol. 13(6), 1243–1259 (2014)
Seyedsalehi, S., Zhang, L., Choi, J., Baek, S.: Prior distributions of material parameters for Bayesian calibration of growth and remodeling computational model of abdominal aortic wall. J. Biomech. Eng. 137(10), 101001 (2015)
Soares, J.S., Sacks, M.S.: A triphasic constrained mixture model of engineered tissue formation under in vitro dynamic mechanical conditioning. Biomech. Model. Mechanobiol. 15(2), 293–316 (2016)
Soltz, M.A., Ateshian, G.A.: Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J. Biomech. 31(10), 927–934 (1998)
Soltz, M.A., Ateshian, G.A.: Interstitial fluid pressurization during confined compression cyclical loading of articular cartilage. Ann. Biomed. Eng. 28(2), 150–159 (2000)
Timoshenko, S., Goodier, J.N.: Theory of Elasticity, 3rd edn. McGraw-Hill, New York (1970)
Tinoco, I., Sauer, K., Wang, J.C.: Physical Chemistry: Principles and Applications in Biological Sciences, 3rd edn. Prentice-Hall, Englewood Cliffs (1995)
Torzilli, P., Askari, E., Jenkins, J.: Water content and solute diffusion properties in articular cartilage. In: Biomechanics of Diarthrodial Joints, vol. I, pp. 363–390. Springer, Berlin (1990)
Urban, J.P., Maroudas, A.: Swelling of the intervertebral disc in vitro. Connect. Tissue Res. 9(1), 1–10 (1981)
Valentín, A., Holzapfel, G.A.: Constrained mixture models as tools for testing competing hypotheses in arterial biomechanics: a brief survey. Mech. Res. Commun. 42, 126–133 (2012)
Vernerey, F.J., Farsad, M.: A constrained mixture approach to mechano-sensing and force generation in contractile cells. J. Mech. Behav. Biomed. Mater. 4(8), 1683–1699 (2011)
Wagenseil, J.E.: A constrained mixture model for developing mouse aorta. Biomech. Model. Mechanobiol. 10(5), 671–687 (2011)
Wan, W., Hansen, L., Gleason, R.L. Jr.: A 3-d constrained mixture model for mechanically mediated vascular growth and remodeling. Biomech. Model. Mechanobiol. 9(4), 403–419 (2010)
Wang, J., Zhou, B., Liu, X.S., Fields, A.J., Sanyal, A., Shi, X., Adams, M., Keaveny, T.M., Guo, X.E.: Trabecular plates and rods determine elastic modulus and yield strength of human trabecular bone. Bone 72, 71–80 (2015)
Weinans, H., Huiskes, R., Grootenboer, H.J.: The behavior of adaptive bone-remodeling simulation models. J. Biomech. 25(12), 1425–1441 (1992)
Wineman, A.: On the mechanics of elastomers undergoing scission and cross-linking. Int. J. Adv. Eng. Sci. Appl. Math. 1(2–3), 123–131 (2009)
Wong, M., Ponticiello, M., Kovanen, V., Jurvelin, J.S.: Volumetric changes of articular cartilage during stress relaxation in unconfined compression. J. Biomech. 33(9), 1049–1054 (2000)
Wu, J., Shadden, S.C.: Stability analysis of a continuum-based constrained mixture model for vascular growth and remodeling. Biomech. Model. Mechanobiol. 15(6), 1669–1684 (2016)
Zeinali-Davarani, S., Wang, Y., Chow, M.-J., Turcotte, R., Zhang, Y.: Contribution of collagen fiber undulation to regional biomechanical properties along porcine thoracic aorta. J. Biomech. Eng. 137(5), 051001 (2015)
Acknowledgements
Research reported in this publication was supported by the National Institutes of Health under Award Number R01 GM083925. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nims, R.J., Ateshian, G.A. Reactive Constrained Mixtures for Modeling the Solid Matrix of Biological Tissues. J Elast 129, 69–105 (2017). https://doi.org/10.1007/s10659-017-9630-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10659-017-9630-9