Abstract
Grape phylloxera (Daktulosphaira vitifoliae Fitch) is a serious global pest in viticulture. The insects are sedentary feeders and require a gall to feed and reproduce. The insects induce their feeding site within the meristematic zone of the root tip, where they stay attached, feeding both intra- and intercellularly, and causing damage by reducing plant vigour. Several changes in cell structure and composition, including increased cell division and tissue swelling close to the feeding site, cause an organoid gall called a nodosity to develop. Because alpha expansin genes are involved in cell enlargement and cell wall loosening in many plant tissues it may be anticipated that they are also involved in nodosity formation. To identify expansin genes in Vitis vinifera cv. Pinot noir, we mined for orthologues genes in a comparative analysis. Eleven putative expansin genes were identified and shown to be present in the rootstock Teleki 5C (V. berlandieri Planch. x V. riparia Michx.) using specific PCR followed by DNA sequencing. Expression analysis of young and mature nodosities and uninfested root tips were conducted via quantitative real time PCR (qRT-PCR). Up-regulation was measured for three putative expansin genes (VvEXPA15, -A17 and partly -A20) or down-regulation for three other putative genes (VvEXPA7, -A12, -A20) in nodosities. The present study clearly shows the involvement of putative expansin genes in the phylloxera–root interaction.
Similar content being viewed by others
Introduction
Grape phylloxera (Daktulosphaira vitifoliae Fitch) (Homoptera: Phylloxeridae) cause substantial damage to susceptible varieties of Vitis spp. by organoid root-gall formation, known as nodosities and/or tuberosities. The insects induce a feeding site within the meristematic zone of the root tip, where they stay attached to the root, feeding both inter- and intracellularly (Hofmann 1957) and inducing changes in the uptake and transportation of water, minerals and assimilates (Porten and Huber 2003). Damage is caused by reducing plant vigour, although secondary soil-borne infections by saprophytes are also thought to cause substantial harm to plants so attacked (Hoffmann et al. 2011). The majority of commercial rootstocks employed in viticulture tolerate phylloxera populations on nodosities; however, due to changed environmental conditions and extensive vineyard management, increasing phylloxera densities are seen to cause grapevine stress in viticulture regions worldwide (Kocsis et al. 1999; Granett et al. 2001; Forneck et al. 2001a). Nodosity induction is thought to be triggered by the phylloxera’s saliva (Miles 1968) which induces, besides several changes in cell structure (Niklowitz 1955), composition (Steffan and Rilling 1981; Schaefer 1985) and volatile metabolomics (Lawo et al. 2011a), an increased cell growth resulting in hook-shaped tissue swelling close to the feeding site (Forneck et al. 2002; Kellow et al. 2004).
Involvement of expansin genes in cell enlargement and cell wall loosening via non-enzymatic mechanisms has been reported in many plant tissues, for example, during fruit softening, pollen tube invasion, root hair growth and organ abscission (Cosgrove 2000). Expansin genes are also involved in nematode feeding site formation upon cyst (Globodera spp., Heterodera spp.) and root-knot nematode (Meloidogyne spp.) attack in tomato (Gal et al. 2006; Griesser and Grundler 2008), Arabidopsis thaliana (Jammes et al. 2005; Wieczorek et al. 2006), soybean (Ibrahim et al. 2011) and peanut (Tirumalaraju et al. 2011). In tomato the expansin genes LeEXPA2, -A5 and -A11 were up-regulated upon cyst nematode attack (Griesser and Grundler 2008), whereas in A. thaliana, up-regulation of expansin genes was observed for AtEXPA1, -A15 and -B3 genes upon root-knot nematode attack (Jammes et al. 2005) and for AtEXPA1, -A3, -A6, -A8, -A10, -A16 and -B3 genes upon cyst nematode attack (Wieczorek et al. 2006). Down-regulation of expansin genes expression has also been shown for AtEXPA7 and -A18 genes in the feeding structure (syncytia) of cyst nematodes (Wieczorek et al. 2006). Two major gene families of expansin genes are described in the literature: the α-expansins (EXPA) and the β-expansins (EXPB) (Kende et al. 2004). For A. thaliana, 26 EXPA, five EXPB and four expansin-like proteins have now been characterized (Sampedro and Cosgrove 2005).
For Vitis spp., no extended analysis of expansin gene families has been conducted to date. Studies only refer to expansin genes in Vitis spp. during grape berry development (Deluc et al. 2007; Ishimaru et al. 2007; Schlosser et al. 2008) or during the ripening phase (Chervin et al. 2008). Knowledge on the regulation of expansin genes is still scarce, but in many cases expression is regulated by plant hormones (e.g. auxins) or abiotic stress (e.g. Bray 2004; Cosgrove 2005; Lee et al. 2005). It has been recently demonstrated that nematodes secrete proteins similar to plant expansin gene products (Wieczorek et al. 2006) and that the saliva of aphids contain proteins (enzymes) able to break down cell walls (Carolan et al. 2011). Whilst phylloxera saliva studies have not yet been performed, aphid secretions do nevertheless contain expansin-like and other cell-wall-loosening proteins that may complement the establishment of the feeding site developed by the host plant.
In the present study, we investigated whether the expression of putative expansin genes are specifically regulated during nodosity formation in roots of a commercial rootstock Teleki 5C (V. berlandieri Planch. x V. riparia Michx.) and thus are an essential part of the herbivore–plant interaction. To illustrate the role of expansin genes in this interaction, the following steps were performed: (1) in silico analysis to identify putative expansin genes in Vitis vinifera cv. Pinot; (2) similarity studies to analyze candidate expansin gene sequences of V. vinifera cv. Pinot noir and the rootstock Teleki 5C (V. berlandieri x V. riparia); and (3), quantitative real-time PCR (qRT-PCR) studies to gain detailed understanding of the expression of candidate expansin genes during nodosity formation.
Material and methods
Biological material
Phylloxera (Daktulosphaira vitifoliae)
Insects were collected in Grosshoeflein, Austria in 2007. A single asexually reproducing founder phylloxera lineage was established and subsequently maintained in a greenhouse without further addition of field-collected material. The line was reared on leaves and roots of caged potted plants of the rootstock Teleki 5C (V. berlandieri Planch. x V. riparia Michx.) and confirmed through microsatellite genotyping according to Vorwerk and Forneck (2006) and Vorwerk et al. (2007) (data not shown). Plants were watered and fertilized [5 ml/3 L; FERTY Spezial Mega (PLANTAN, Germany)] as required.
Root tissue collection
Young nodosities [infested by one 3rd nymphal stage phylloxera using the nomenclature for phylloxera as suggested by Forneck and Huber (2009)] were collected from greenhouse cultures during June 2009 to verify the expression of putative expansin genes in the rootstock Teleki 5C (for details see Lawo et al. 2011b).
For quantitative real-time (qRT-) PCR studies, young nodosities (infested by one 2nd or 3rd nymphal stage phylloxera; Ny), mature nodosities (infested by one adult phylloxera producing eggs; Nm) and uninfested root tips (length ca. 1.5 cm) from uninfested plants were collected from the rootstock Teleki 5C from June–October 2010 produced from several independent inoculations of the same asexual phylloxera lineage in the greenhouse. A rearing system was performed according to Lawo et al. (2011b). Prior to freezing, the galls were separated into the basipetal body of the gall/root and the apical tip. Samples were stored at −80 °C until extraction of RNA.
Identification and verification of putative expansin genes
According to the current scientific literature, four expansin genes have been identified in Vitis spp. [Vexp-1 (Q84UT0), Vexp-2 (Q84US9), Vexp-3 (Q84US8), Vexp-4 (Q84US7)], whilst a fifth gene is essentially similar to an expansin gene [tam_exps (E3WHD4)]. A BLAST search with the above gene sequences revealed three different predicted expansin genes from A. thaliana, namely AtEXPA2, -A8 and -A15 (score: 394–332; E-value: 1e-91–3e−93) (data not shown). To identify further putative expansin genes in Vitis spp., the amino acid sequences of expansin genes from A. thaliana were taken as a template (Cosgrove 2006) and BLAST search within the V. vinifera genome database Genoscope performed (Jaillon et al. 2007). Orthologous genes for AtEXPA4, -A7, -A8, -A11, -A12, -A15, -A17, -A20, -A23, -B2 and -B3 were identified. A phylogenetic tree produced in order to give an overview of the genetic relationship among the genes identified is given in the supplemental material (Supplementary Figure 1). For the verification of the expression of identified putative expansin genes in the rootstock Teleki 5C, a specific polymerase chain reaction (PCR) followed by sequencing (Agowa, Berlin, Germany) was performed. DNA from V. vinifera Pinot noir Clone 18Gm served as a control to ensure primer binding.
Gene expression analyzes
Total RNA was extracted from individual samples of young or mature nodosity bodies or uninfested root tips and from samples of mature nodosity apex vs. root apex, using a modified RNeasy Plant Mini Kit (Qiagen, Hilden, Germany) as described in Lawo et al. (2011b), including an additional step for DNA digestion (RNase-Free DNase Set, Qiagen, Germany). Each individual sample included between 16 and 24 tissue pieces. Concentration and integrity of the isolated RNA was determined using a NanoDrop 2000/2000c Spectrophotometer (Thermo Fisher Scientific, USA). Samples with a 260/230 ratio below 1.4 were checked on a gel to exclude any RNA degradation. A subsequent reverse transcription was performed using a QuantiTec Reverse Transcription kit (Qiagen, Hilden, Germany). 500 ng RNA was used for reverse transcription and qRT-PCR reactions were performed using the Rotorgene cycler (Qiagen, Hilden, Germany) employing KAPA SYBR FAST qPCR Universal (Peqlab, Erlangen, Germany) as a detector agent. All samples were pipetted in duplicates, whilst all primers were tested for their efficiency prior to analysis by conducting standard curves for four step template dilutions. Efficiencies were calculated using the formula: \( \mathrm{E}=\left[ {1{0^{{\left( {{-1 \left/ {\mathrm{slope}} \right.}} \right)}}}} \right]-1 \). Primers with efficiencies ≪ 87 % were discarded for further studies. A concentration of 200 nM was used for all primers (Table 1). Cycling conditions were: one cycle for 4 min at 95 °C, 35 cycles for 5 s at 95 °C, 20 s at 60 °C, 5 s at 72 °C and 10 s at 75 °C, where the fluorescence signal was measured. Dissociation curves were performed with continuous fluorescence acquisition between 70 °C to 95 °C. Blanks were accomplished for each primer pair per run. Quantitative fold changes (FC) were calculated using the 2−ddCt formula (Livak and Schmittgen 2001). Thus a FC of 1.0 indicates no gene regulation, whether a FC of two indicates a two-fold expression and one of 0.5 half of the gene expression. To determine putative expansin gene expression, three to four independent samples were analyzed as biological replicates. The relative expression (fold change) of putative expansin genes in nodosities compared to uninfested root tips was normalized to the expression of two reference genes as suggested by Pfaffl (2001) [actin (GSVIVT00034893001) and ubiquitin 1 (GSVIVT00037199001)] chosen in preliminary studies (data not shown). Pretests were performed with two (mature nodosities and uninfested root tips) or one (young nodosities) sample. Additional analyses (resulting in four biological replicates in total) were performed with genes showing a FC either ≪ 0.4 or ≫ 2.5 in at least two of the four treatments.
Data analysis
FC-values from qRT-PCR data were calculated to determine changes in expression. T-tests were used for exploratory assessment of changes from 1.0 following calculation of unadjusted two-sided 95 % confidence intervals and respective p-values. Statistical analyses were conducted using the software package SPSS, version 16 (SPSS Incorporation). As the sample size (N) was limited, data was not corrected; thus the study has an exploratory character.
Results
Identification and verification of putative expansin genes
Following in silico studies, eleven putative expansin genes were identified in V. vinifera cv. P. noir homologous in terms of sequence with those from thaliana (Supplementary Figure 1). Nine of these genes were verified in the rootstock Teleki 5C via PCR-based sequencing (VvEXPA4, -A7, -A8, -11, -A12, -A15, -A17, -A23, -B2). Sequences showed 98–100 % similarity when compared to the databank from Genoscope (Jaillon et al. 2007) (data not shown). In the following (as below), putative expansin genes from the rootstock Teleki 5C were pooled under the abbreviation “VvEXP” due to the fact that no further functional or genomic studies on this genes have been performed to indicate the similarity (if any) to V. vinifera expansin genes.
Expression of putative expansin genes
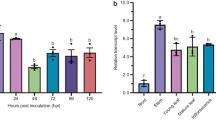
Our qRT-PCR study revealed an involvement of the putative expansin genes VvEXPA7, -A12, -A15, -A17, -A20 and -B2 in phylloxera-root interaction expressed either via up- (quantitative FC >2) or down-regulation (quantitative FC < 0.5 [a schematic overview of the regulation is given in Fig. 1]). Significant up-regulation was observed for the putative expansin genes VvEXPA15 (P = 0.035) and -A17 (P = 0.005) in the bodies of mature nodosity as well as for the putative expansin gene VvEXPA20 at the tips of mature nodosities (P = 0.036). Significant down-regulation was calculated for the putative expansin genes VvEXPA7 in all treatments (body of young nodosities: P = 0.045; body of mature nodosities: P = 0.0004, tips of mature nodosities: P = 0.030) and further down-regulation was observed for the putative expansin gene VvEXPA20 in the body of mature nodosities (P = 0.011) in addition to VvEXPA12 in mature nodosity tips (P = 0.024).
Schematic drawing of young (a) and mature (b) nodosities showing which putative expansin genes were up- or down-regulated after a phylloxera attack and whether this occurred in the nodosity body and/or the root tip. All putative expansin genes start with VvEXP-. Stars indicate significance for a certain putative expansin gene. No putative expansin genes were measured in the tips of young nodosities (N.A.)
As gene expression for VvEXPB2 was either up- or down-regulated within the same treatment and a high standard error was calculated for those data points, results are not discussed further for this gene. An overview excluding VvEXPB2 is given in Fig. 2 based on the calculated FCs.
Expression changes of putative expansin genes [relative fold change (FC) ± se] in young and mature nodosity bodies and mature nodosity tips. At a FC of 1.0, no gene regulation occurs (black line), an FC of 2.0 indicates that a doubling of gene expression occurs (dashed line), whilst an FC of 0.5 indicates that only half of the potential gene expression occurs (dotted line). Values above 1.0 indicate up-regulation, values below 1.0 down-regulation in gene expression. Treatments are in the same order for all genes. N = 3–4
Discussion
This study clearly shows that putative expansin genes are involved in grape root phylloxera-interaction, with up- as well as down-regulated genes. Three putative expansin genes (VvEXPA15, -A17 and partly -A20) were up-regulated in nodosities whilst three putative genes were partly down-regulated in young or mature nodosity bodies or mature nodosity tips (VvEXPA7, -A12, -A20).
Expression profiles in nodosities
Initiation and development of nodosities involves a combination of hypertrophy of cortical cells distal to the feeding site and lack of radial expansion of cortical cells proximal to the feeding site. The active feeding site is located in the upper layers of the root cortex whilst the stylet sheath ends intracellularly apart from the endodermis (Forneck et al. 2002). The nodosity apical end retains the meristematic cell profiles and calyptra but does not show major histological cell modifications, which are mainly hypertrophic due to phylloxera feeding.
By studying young vs. mature nodosities and dividing the latter into basipetal vs. apical ends of the galls, our aim was to search for information regarding the expansin regulation during nodosity growth. Comparing our results between young and mature nodosity bodies, it is clear that more putative expansin genes are expressed over time, resulting in up- as well as down-regulation (young nodosities: down-regulated: VvEXPA7; mature nodosities: down-regulated: VvEXPA7, and A20, up-regulated: VvEXPA15 and -A17). As the body of the root galls enlarge via cell growth (Niklowitz 1955; Forneck et al. 2002), up-regulation in case of mature nodosity bodies seemingly indicates cell-wall loosening and modification—characteristics well known to be associated with expansin genes. A stronger down-regulation of putative expansin genes in mature nodosity bodies compared to young ones is probably a result of plant defence. Indications of plant defence mechanisms in compatible phylloxera-plant reactions were earlier shown by Lawo et al. (2011a) who found that mevalonate and/or alternative isopentenyl pyrophosphate-, the lipoxygenase- (LOX) as well as the phenylpropanoid pathway, are induced as a direct response to phylloxera attack in the nodosity. In this particular paper, it was also shown that volatile profile in young nodosities was less clearly expressed compared to mature nodosities.
The comparison of expression levels of putative expansin genes in the basipetal ends of mature nodosities versus their apical ends showed similar up-regulation only for VvEXPA15 (FC: 4.3 ± 0.89 vs. 4.54 ± 1.19). A significantly stronger down-regulation of the putative expansin gene VvEXPA7 occurs at the base of the root compared to its tip (FC: 0.1 ± 0.05 vs. 0.5 ± 0.13). Selective sampling further also showed that VvEXPA17 was mainly expressed in the basipetal main part of the gall (FC: 2.7 ± 0.23 vs. 1.1 ± 0.36) and for the putative expansin gene VvEXPA20, a down-regulation in nodosity main parts was observed, whereas significant up-regulation was measured in the tips (FC: 0.4 ± 0.01 vs. 1.7 ± 0.20).
Comparison of root galls between nematodes and phylloxera
Gall formation of nematodes and insects has been contrasted for a long time, although gall induction in plants through pathogen involvement may involve similar host pathways or that host responses involve common plant regulatory cascades (e.g. Bird and Kaloshian 2003). The majority of studies on expansin expression in plants were performed using nematodes, either root-knot nematodes which form galls or cyst nematodes which form syncytia. Whether cyst nematodes establish their feeding site via a single root cell which then fuses with neighbouring cells is still not clear (Wyss and Grundler 1992), although root-knot nematodes seem to use 5–7 parenchymatic cells to establish their so-called giant cells (Wyss et al. 1992). In case of nodosity formation, root cells close to the phylloxera’s feeding site become polyploid, whereas those at opposite to the feeding site enlarge and are filled with starch granules (summarised by Forneck et al. 2002).
Several previous studies are in agreement with our findings in reporting up-regulation of the expansin gene AtEXPA15 (referring to VvEXPA15) in syncytium formation (Wieczorek et al. 2006) as well as upon root-knot nematode attack (Jammes et al. 2005). Down regulation was observed for the putative expansin genes VvEXPA7 and partly VvEXPA12 and -A20 in nodosity bodies and/or tips (these results). Similar results were shown upon cyst nematode attack in syncytia only for AtEXPA7 in A. thaliana (Wieczorek et al. 2006; Szakasits et al. 2009).
Furthermore, upon comparing our findings with a phylogenetic tree based on the protein sequences of A. thaliana and tomato (Griesser and Grundler 2008), none of the putative expansin genes involved in nodosity formation in tomato were found to be regulated under root-knot nematode attack. This, together with the difference in gall formation as discussed above, provides strong evidence that differently regulated expansin genes are triggered due to different herbivore–plant interactions. However, an up-regulation of expansin genes is not only limited to gall-forming pests. Thus studies by Divol et al. (2005) have reported an increased expression of two expansin genes in celery after phloem feeding of the peach-potato aphid, Myzus persicae (Sulzer). In contrast, Kempema et al. (2007) reported the down-regulation of three expansin genes due to phloem-feeding whitefly nymphs (Bemesia tabaci Gennadius) on A. thaliana. Such examples infer that expansin genes are not only associated with cell growth and expansion but that their function is much more complex.
Several studies show that besides expansin genes, other genes involved in cell wall modification such as glucanases, pectinases or cellulases are up-regulated as a result of root-knot nematode attack (Caillaud et al. 2008) as well as the gene family of endo-1,4-β-glucanases upon cyst nematode attack (Wieczorek et al. 2008). So far no detail studies related to this field have been conducted in relation to nodosity formation by phylloxera. Even so, earlier studies have provided some evidence of physiological changes in nodosities upon attack by this insect (e.g. Forneck et al. 2001b; Kellow et al. 2004; Du et al. 2011) arguing that not only genes involved in cell wall modification are affected. Thus, further enzymatic changes expressed in increased activity of peroxidase, acidic phosphatase and leucine aminopeptidase have also been shown upon phylloxera attack (Forneck et al. 2002). Other studies have reported the accumulation of free amino acids and amides in nodosities compared to uninfested roots (Kellow et al. 2004) and recently a change in endogenous hormone levels in nodosities over time was measured (Du et al. 2011).
In this study we have shown that expansin genes are specifically regulated in the phylloxera–root interaction. The studies of Lawo et al. (2011b) are consistent with our present findings of an up-regulation of the putative expansin gene VvEXPA15 as well as a down-regulation of genes VvEXPA7 and -A12. In our present study, even when complete nodosities of different age were compared to control roots, a similar expression pattern was revealed. Based on this plus the involvement of these genes upon nematode attack, we conclude that the putative expansin gene VvEXPA15 is essential during gall formation processes and further, that the genes VvEXPA7, -12, -20 and -17 are involved in nodosity formation. To further analyze and complement gene function, studies are necessary with adequate transgenic grapevines (since there are no knock-out mutants available) to prove expansin—effects on the gall formation upon phylloxera infection. To localize the expression of expansin genes in nodosities by in situ hybridization (e.g. Vorwerk et al. 2008) has been shown to be efficient and may further enhance our knowledge on the nodosity development.
References
Bird, D. K., & Kaloshian, I. (2003). Are roots special? Nematodes have their say. Physiological and Molecular Plant Pathology, 62, 115–123.
Bray, E. A. (2004). Genes commonly regulated by water-deficit stress in Arabidopsis thaliana. Journal of Experimental Botany, 55(407), 2331–2341.
Caillaud, M. C., Dubreuil, G., Quentin, M., Perfus-Barbeoch, L., Lecomte, P., de Almeida Engler, J., et al. (2008). Root-knot nematodes manipulate plant cell functions during a compatible interaction. Journal of Plant Physiology, 165(1), 104–113.
Carolan, J. C., Caragea, D., Reardon, K. T., Mutti, N. S., Dittmer, N., Pappan, K., et al. (2011). Predicted effector molecules in the salivary secretome of the Pea Aphid (Acyrthosiphon pisum): a dual transcriptomic/proteomic approach. Journal of Proteome Research, 10, 1505–1518.
Chervin, C., Tira-Umphon, A., Terrier, N., Zouine, M., Severac, D., & Roustan, J. P. (2008). Stimulation of the grape berry expansion by ethylene and effects on related gene transcripts, over the ripening phase. Physiologia Plantarum, 134, 534–546.
Cosgrove, D. J. (2000). Loosening of plant cell walls by expansins. Nature, 407, 321–326.
Cosgrove, D. J. (2005). Growth of the plant cell wall. Nature Reviews Molecular Cell Biology, 6, 850–861.
Cosgrove, D.J. (2006). http://homes.bio.psu.edu/expansins/
Deluc, L., Grimplet, J., Wheatley, M., Tillett, R., Quilici, D., Osborne, C., et al. (2007). Transcriptomic and metabolite analyses of Cabernet Sauvignon grape berry development. BMC Genome, 8, 429. doi:10.1186/1471-2164-8-429.
Divol, F., Vilaine, F., Thibivilliers, S., Amselem, J., Palauqui, J. C., Kusiak, C., et al. (2005). Systemic response to aphid infestation by Myzus persicae in the phloem of Apium graveolens. Plant Molecular Biology, 57, 517–540.
Du, Y. P., Wang, Z. S., & Zhai, H. (2011). Grape root cell features related to phylloxera resistance and changes of anatomy and endogenous hormones during nodosity and tuberosity formation. Australian Journal of Grape and Wine Research, 17, 291–297.
Forneck, A., & Huber, L. (2009). (A)sexual reproduction—a review of life cycles of grape phylloxera, Daktulosphaira vitifoliae. Entomologia Experimentalis et Applicata, 131(1), 1–10.
Forneck, A., Walker, M. A., Blaich, R., Yvon, M., & Leclant, F. (2001a). Interaction of phylloxera (Daktulosphaira vitifoliae Fitch) with grape (Vitis spp.) in simple isolation chambers. American Journal of Enology and Viticulture, 52(1), 28–34.
Forneck, A., Walker, M. A., & Blaich, R. (2001b). Ecological and genetic aspects of grape phylloxera Daktulosphaira vitifoliae (Hemiptera: Phylloxeridae) performance on rootstock hosts. Bulletin of Entomological Research, 91(6), 445–451.
Forneck, A., Kleinmann, S., & Blaich, R. (2002). Histochemistry and anatomy of phylloxera (Daktulosphaira vitifoliae) induced galls (nodosities) on young roots of grape vine (Vitis spp.). Vitis, 41, 93–97.
Gal, T., Aussenberg, E., Burdman, S., Kapulnik, Y., & Koltai, H. (2006). Expression of a plant expansin is involved in the establishment of root knot nematode parasitism in tomato. Planta, 224, 155–162.
Granett, J., Walker, M. A., Kocsis, L., & Omer, A. D. (2001). Biology and management of grape phylloxera. Annual Review of Entomology, 46, 387–412.
Griesser, M., & Grundler, F. M. W. (2008). Quantification of tomato expansins in nematode feeding sites of cyst and root-knot nematodes. Journal of Plant Diseases and Protection, 115, 263–272.
Hofmann, E. L. (1957). Die Histologie der Nodositäten verschiedener Rebensorten bei Reblausbefall. Vitis, 1, 125–141.
Hoffmann, M., Ruehl, E. H., Huber, L., Eisenbeis, G., & Kirchmair, M. (2011). A scanner based approach to assess grape root infesting parasites in field. Acta Horticulturae, 904, 101–109.
Ibrahim, H., Hosseini, P., Alkharouf, N., Hussein, E., Gamal El-Din, A. E. K., Aly, M., et al. (2011). Analysis of gene expression in soybean (Glycine max) roots in response to the root knot nematode Meloidogyne incognita using microarrays and KEGG pathways. BCM Genomics, 12(1), 220.
Ishimaru, M., Smith, D. L., Gross, K. C., & Kobayashi, S. (2007). Expression of three expansin genes during development and maturation of Kyoho grape berries. Journal of Plant Physiology, 164, 1675–1682.
Jaillon, O., Aury, J. M., Noel, B., Policriti, A., Clepet, C., Casagrande, A., et al. (2007). The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature, 449, 463–467.
Jammes, F., Lecomte, P., De Almeida-Engler, J., Bitton, F., Marin-Magniette, M. L., Renou, J. P., et al. (2005). Genome-wide expression profiling of the host response toroot-knot nematode infection in Arabidopsis. The Plant Journal, 44, 447–458.
Kellow, A. V., Sedgley, M., & Van Heeswijck, R. (2004). Interaction between Vitis vinifera and grape phylloxera: Changes in root tissue during nodosity formation. Annals of Botany, 93, 581–590.
Kempema, L. A., Cui, X., Holzer, F. M., & Walling, L. L. (2007). Arabidopsis transcriptome changes in response to phloem-feeding silverleaf whitefly nymphs. Similarities and distinctions in responses to aphids. Plant Physiology, 143, 849–865.
Kende, H., Bradford, K., Brummell, D., Cho, H.-T., Cosgrove, D., Fleming, A., et al. (2004). Nomenclature for members of the expansin superfamily of genes and proteins. Plant Molecular Biology, 55(3), 311–314.
Kocsis, L., Granett, J., Walker, M. A., Lin, H., & Omer, A. D. (1999). Grape phylloxera populations adapted to Vitis berlandieri x V. riparia rootstocks. American Journal of Enology and Viticulture, 50(1), 101–106.
Lawo, N. C., Weingart, G., Schuhmacher, R., & Forneck, A. (2011a). The volatile metabolome of grapevine roots: First insights into the metabolic response upon phylloxera attack. Plant Physiology and Biochemistry, 49(9), 1059–1063.
Lawo, N. C., Maleno, F., Griesser, M., & Forneck, A. (2011b). Expression of reference genes in nodosities and their application. Acta Horticulturae, 904, 77–84.
Lee, B.-H., Henderson, D. A., & Zhu, J. K. (2005). The Arabidopsis cold-responsive transcriptome and its regulation by ICE1. The Plant Cell, 17, 3155–3175.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods, 25(4), 402–408.
Miles, P. W. (1968). Studies on the salivary physiology of plant-bugs: Experimental induction of galls. Journal of Insect Physiology, 14(1), 97–100.
Niklowitz, W. (1955). Histologische Studien an Rebalusgallen. Phytopathologische Zeitschrift, 24, 299–340.
Pfaffl, M. W. (2001). A new mathematical model for relative quantification real-time RT-PCR. Nucleic Acids Research, 29, 2002–2007.
Porten, M., & Huber, L. (2003). An assessment method for the quantification of Daktulosphaira vitifoliae (Fitch) (Hom., Phylloxeridae) populations in the field. Journal of Applied Entomology, 127(3), 157–162.
Sampedro, J., & Cosgrove, D. J. (2005). The expansin superfamily. Genome Biology, 6, 242–250.
Schaefer, H. (1985). Stoffwechselunterschiede zwischen Reblauswurzelgallen und gesunden Rebenwurzeln. Weinwissenschaft, 4, 219–226.
Schlosser, J., Olsson, N., Weis, M., Reid, K., Peng, F., Lund, S., et al. (2008). Cellular expansion and gene expression in the developing grape (Vitis vinifera L.). Protoplasma, 232, 255–265.
Steffan, H., & Rilling, G. (1981). Der Einfluss von Blatt- und Wurzelgallen der Reblaus (Dactylosphaera vitifolii Shimer) auf das Verteilungsmuster der Assimilate in Reben (V. rupestris 187G). Vitis, 20, 146–155.
Szakasits, D., Heinen, P., Wieczorek, K., Hofmann, J., Wagner, F., Kreil, D. P., et al. (2009). The transcriptome of syncytia induced by the cyst nematode Heterodera schachtii in Arabidopsis roots. The Plant Journal, 57, 771–784.
Tirumalaraju, S. V., Jain, M., & Gallo, M. (2011). Differential gene expression in roots of nematode-resistant and -susceptible peanut (Arachis hypogaea) cultivars in response to early stages of peanut root-knot nematode (Meloidogyne arenaria) parasitization. Journal of Plant Physiology, 168(5), 481–492.
Vorwerk, S., & Forneck, A. (2006). Reproductive mode of grape phylloxera (Daktulosphaira vitifoliae, Homptera: Phylloxeridae) in Europe: molecular evidence for predominantly asexual populations and a lack of gene flow between them. Genome, 49(6), 678–687.
Vorwerk, S; Forneck, A. Analysis of genetic variation within clonal lineages of grape phylloxera (Daktulosphaira vitifoliae Fitch) using AFLP fingerprinting and DNA sequencing. (2007). Genome 50(7), 660–667.
Vorwerk, S., Kosanetzki, K., Blaich, K., & Forneck, A. (2008). Application of current in situ hybridization techniques for grape phylloxera (Daktulosphaira vitifoliae, Fitch) and grapevine (Vitis spp. L.). Vitis, 47(2), 113–116.
Wieczorek, K., Golecki, B., Gerdes, L., Heinen, P., Szakasits, D., Durachko, D. M., et al. (2006). Expansins are involved in the formation of nematode-induced syncytia in roots of Arabidopsis thaliana. The Plant Journal, 48, 98–112.
Wieczorek, K., Hofmann, J., Bloechl, A., Szakasits, D., Bohlmann, H., & Grundler, F. M. W. (2008). Arabidopsis endo-1,4-b-glucanases are involved in the formation of root syncytia induced by Heterodera schachtii. The Plant Journal, 53, 336–351.
Wyss, U., & Grundler, F. M. W. (1992). Feeding behavior of sedentary plant parasitic nematodes. Netherlands Journal of Plant Pathology, 98(Suppl. 2), 165–173.
Wyss, U., Grundler, F. M. W., & Muench, A. (1992). The parasitic behaviour of second-stage juveniles of Meloidogyne incognita in roots of Arabidopsis thaliana. Nematologica, 38, 98–111.
Acknowledgements
The authors thank the Austrian Science Fund (FWF) for the financially supporting this research project (21203-B16) and Sarah Bardakji and Vanessa Loiacono for their technical assistance and Dr. K. Wieczorek and Dr. H. Loxdale and two anonymous reviewers for discussion and careful revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
No caption (PDF 111 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Lawo, N.C., Griesser, M. & Forneck, A. Expression of putative expansin genes in phylloxera (Daktulosphaira vitifoliae Fitch) induced root galls of Vitis spp.. Eur J Plant Pathol 136, 383–391 (2013). https://doi.org/10.1007/s10658-013-0173-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-013-0173-z