Abstract
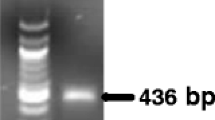
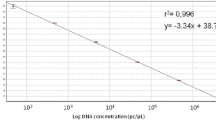
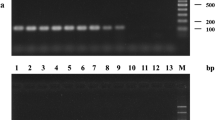
Stagonosporopsis andigena and S. crystalliniformis are serious foliage pathogens on potato (Solanum tuberosum) and tomato (Solanum lycopersicum). As both species have been recorded only in the Andes area, S. andigena is listed as an A1 quarantine organism in Europe. The actin region of isolates of Stagonosporopsis and allied species of Boeremia, Didymella, Peyronellaea and Phoma was amplified using generic primers. DNA sequence differences of the actin gene were utilised to develop species-specific real-time (TaqMan) PCR assays for the detection of S. andigena and S. crystalliniformis in leaves of potato or tomato. The specificity of the TaqMan PCR assays was determined on genomic DNA extracted from two S. andigena and two S. crystalliniformis isolates and 16 selected isolates of Stagonosporopsis, Phoma and Boeremia, which are the closest relatives. The validation of the methods developed included the DNA extraction and the TaqMan PCR assays. The performance criteria specificity, analytical sensitivity, reproducibility, repeatability and robustness of the TaqMan PCR assays demonstrated the reliability of both methods for the detection of S. andigena and S. crystalliniformis in leaf material. The TaqMan PCR assays were tested on symptomatic leaves of potato and tomato that were obtained after artificial inoculation of detached leaves with both pathogens under quarantine conditions. In the artificial inoculation experiments both S. andigena and S. crystalliniformis caused leaf infections on potato and tomato.

Similar content being viewed by others
References
Arzanlou, M., Kema, G. H. J., Waalwijk, C., Carlier, J., De Vries, I., Guzman, M., Vargas, M. A., Helder, J., & Crous, P. W. (2009). Molecular diagnostics in the Mycosphaerella leaf spot disease complex of banana and for Radopholus similis. Acta Horticulturae, 828, 237–244.
Aveskamp, M. M., De Gruyter, J., & Crous, P. W. (2008). Biology and systematics of the genus Phoma: a complex genus of major quarantine significance. Fungal Diversity, 31, 1–18.
Aveskamp, M. M., Verkley, G. J. M., De Gruyter, J., Murace, M. A., Perelló, A., Woudenberg, J. H. C., Groenewald, J. Z., & Crous, P. W. (2009a). DNA phylogeny reveals polyphyly of Phoma section Peyronellaea and multiple taxonomic novelties. Mycologia, 101, 363–382.
Aveskamp, M. M., Woudenberg, J. H. C., De Gruyter, J., Turco, E., Groenewald, J. Z., & Crous, P. W. (2009b). Development of taxon-specific SCAR markers based on actin sequences and DAF: a case study in the Phoma exigua species complex. Molecular Plant Pathology, 10, 403–414.
Aveskamp, M. M., De Gruyter, J., Woudenberg, J. H. C., Verkley, G. J. M., & Crous, P. W. (2010). Highlights of the Didymellaceae: a polyphasic approach to characterise Phoma and related pleosporalean genera. Studies in Mycology, 65, 1–60.
Balmas, V., Scherm, B., Ghignone, S., Salem, A. O. M., Cacciola, S. O., & Migheli, Q. (2005). Characterisation of Phoma tracheiphila by RAPD-PCR, microsatellite-primed PCR and ITS rDNA sequencing and development of specific primers for in planta PCR detection. European Journal of Plant Pathology, 111, 235–247.
Boerema, G. H., De Gruyter, J., & Noordeloos, M. E. (1995). New names in Phoma. Persoonia, 16, 131.
Boerema, G. H., De Gruyter, J., & Noordeloos, M. E. (1997). Contributions towards a monograph of Phoma (Coelomycetes)—IV. Section Heterospora: Taxa with large sized conidial dimorphs, in vivo sometimes as Stagonosporopsis synanamorphs. Persoonia, 16, 335–371.
Boerema, G. H., De Gruyter, J., Noordeloos, M. E., & Hamers, M. E. C. (2004). Phoma identification manual. Differentiation of specific and infra-specific taxa in culture. Wallingford: CABI Publishing.
Carbone, I., & Kohn, L. M. (1999). A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia, 91, 553–556.
Cline, E. (2011). Phoma andigena. Systematic Mycology and Microbiology Laboratory, ARS, USDA. Retrieved July 1, 2011, from http://nt.ars-grin.gov/sbmlweb/fungi/index.cfm.
Crous, P. W., Verkley, G. J. M., Groenewald, J. Z., & Samson, R. A. (2009). Fungal biodiversity. CBS Laboratory Manual Series. Utrecht: Centraalbureau voor Schimmelcultures.
Cullen, D. W., Toth, I. K., Boonham, N., Walsh, K., Barker, I., & Lees, A. K. (2007). Development and validation of conventional and quantitative polymerase chain reaction assays for the detection of storage rot potato pathogens, Phytophthora erythroseptica, Pythium ultimum, Phoma foveata. Journal of Phytopathology, 155, 309–315.
De Gruyter, J., Aveskamp, M. M., Woudenberg, J. H. C., Verkley, G. J. M., Groenewald, J. Z., & Crous, P. W. (2009). Molecular phylogeny of Phoma and allied anamorph genera: towards a reclassification of the Phoma complex. Mycological Research, 113, 508–519.
De Gruyter, J., Woudenberg, J. H. C., Aveskamp, M. M., Verkley, G. J. M., Groenewald, J. Z., & Crous, P. W. (2010). Systematic reappraisal of species in Phoma section Paraphoma, Pyrenochaeta and Pleurophoma. Mycologia, 102, 1066–1081.
Demontis, M. A., Cacciola, S. O., Orrù, M., Balmas, V., Chessa, V., Maserti, B. E., Mascia, L., Raudino, F., di San, M., Lio, G., & Migheli, Q. (2008). Development of real-time PCR systems based on SYBR® Green I and TaqMan® technologies for specific quantitative detection of Phoma tracheiphila in infected Citrus. European Journal of Plant Pathology, 120, 339–351.
Dutch guideline for the validation of detection and identification methods for plant pathogens and pests. (2010) Retrieved August 31, 2010, from http://www.naktuinbouw.nl/sites/naktuinbouw.eu/files/Dutch%20Validation%20Guideline%20v1.0.pdf.
Huelsenbeck, J. P., & Ronqvist, F. (2001). MRBAYES: Bayesian inference of Phylogenic trees. Bioinformatics, 17, 754–755.
Katoh, K., Asimenos, G., & Toh, H. (2009). Multiple alignment of DNA sequences with MAFFT. In D. Posada (Ed.), Bioinformatics for DNA sequence analysis. Methods in Molecular Biology, 537, 39–64.
Koch, C. A., & Utkhede, R. S. (2004). Development of a multiplex classical polymerase chain reaction technique for detection of Didymella bryoniae in infected cucumber tissues and greenhouse air samples. Canadian Journal of Plant Pathology, 26, 291–298.
Lartey, R. T., Weiland, J. J., Caesar-TonThat, T., & Bucklin-Comiskey, S. (2003). A PCR protocol for rapid detection of Cercospora beticola in sugarbeet tissues. Journal of Sugar Beet Research, 40, 1–10.
Licciardella, G., Grasso, F. M., Bella, P., Cirvilleri, G., Grimaldi, V., & Catara, V. (2006). Identification and detection of Phoma tracheiphila, causal agent of Citrus Mal Secco disease, by real-time polymerase chain reaction. Plant Disease, 90, 1523–1530.
Loerakker, W. M., Navarro, R., Lobo, M., & Turkensteen, L. J. (1986). Phoma andina var. crystalliniformis var. nov., un patógeno nuevo del tomate y de la papa en los Andes. Fitopatología, 21, 99–102.
MacDonald, J. E., White, G. P., & Coté, M. J. (2000). Differentiation of Phoma foveata from P. exigua using a RAPD generated PCR-RFLP marker. European Journal of Plant Pathology, 106, 67–75.
Mumford, R. A., Skelton, A. L., Boonham, N., Postuma, K. I., Kirby, M. J., & Adams, A. N. (2004). The improved detection of Strawberry Crinkle Virus using Real-Time RT-PCR (Taqman®). In R. R. Martin (Ed.), ISHS Xth international symposium on small fruit virus diseases. Valencia, Spain, Acta Horticulturae, 656, 81–86.
Navarro, R. A., & Puerta, O. D. (1981). Presencia de Phoma sp. en tomate (Lycopersicon esculentum). Ascolfi Informa, 7, 32.
Noordeloos, M. E., De Gruyter, J., Van Eijk, G. W., & Roeijmans, H. J. (1993). Production of dendritic crystals in pure cultures of Phoma and Ascochyta and its value as a taxonomic character relative to morphology, pathology and cultural characteristics. Mycological Research, 97, 1343–1350.
OEPP/EPPO. (1984). Data sheets on quarantine organisms No. 141, Phoma andina. Bulletin OEPP/EPPO Bulletin, 14, 45–48.
OEPP/EPPO. (2010). PM 7/98 (1): specific requirements for laboratories preparing accreditation for a plant pest diagnostic activity. Bulletin OEPP/EPPO Bulletin, 40, 5–22.
Otazú, V., Boerema, G. H., Mooi, J. C., & Salas, B. (1979). Possible geographical origin of Phoma exigua var. foveata, the principal causal organism of potato gangrene. Potato Research, 22, 333–338.
Page, R. D. M. (1996). Treeview: an application to display phylogenetic trees on personal computers. Computer Application in the Biosciences, 12, 357–358.
Somai, B. M., Keinath, A. P., & Dean, R. A. (2002). Development of PCR-ELISA for detection and differentiation of Didymella bryoniae from related Phoma species. Plant Disease, 86, 710–716.
Turkensteen, L. J. (1978). Tizón foliar de la papa en el Perú. I, Especies de Phoma asociadas. Fitopatología, 13, 67–69.
Turkensteen, L. J., & Pinedo, H. M. (1982). Didymella bryoniae como patógeno de la papa en las regiones Cálidas del Perú. Fitopatología, 17, 54–55.
Weller, S. A., Elphinstone, J. G., Smith, N. C., Boonham, N., & Stead, D. E. (2000). Detection of Ralstonia solanacearum strains with a quantitative, multiplex, real-time, fluorogenic PCR (TaqMan) assay. Applied Environmental Microbiology, 66, 2853–2558.
White, T. J., Bruns, T., Lee, S., & Taylor, J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In M. A. Innis, D. H. Gelfand, J. J. Sninsky, & T. J. White (Eds.), PCR protocols: a guide to methods and applications (pp. 315–322). San Diego: Academic.
Acknowledgments
The Dutch Ministry of Economics, Innovation and Agriculture has supported this research through an endowment of the FES program ‘Reinforcement Infrastructure Plant Health’.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Gruyter, J., van Gent-Pelzer, M.P.E., Woudenberg, J.H.C. et al. The development of a validated real-time (TaqMan) PCR for detection of Stagonosporopsis andigena and S. crystalliniformis in infected leaves of potato and tomato. Eur J Plant Pathol 134, 301–313 (2012). https://doi.org/10.1007/s10658-012-9990-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-012-9990-8