Abstract
Brassica rapa (chinese cabbage) is one of the main vegetable crops grown in Asian countries. Pectobacterium carotovorum subsp. carotovorum causes severe economic loss in this crop as well as in other Brassica crops through soft rot disease. Cysteine proteases like bromelain, papain or ficin show toxic effects to herbivorous insects and pathogenic bacteria. They have been known to be critical factors in plant defence mechanisms. The current study investigated the effect of bromelain gene (BL1) of pineapple (Ananas comosus L. Merrill) on enhancing resistance to soft rot in transgenic Brassica rapa ‘Seoulbaechu’. Three homozygous T2 lines were inoculated with Pectobacterium carotovorum subsp. carotovorum and BL8-2 line showed the lowest rate of infected leaves (RIL) in both wound inoculation and non-wound inoculation, when the non-infected line showed 100 % RIL in both cases. The highest expression of BL1 gene was also observed in BL8-2 homozygous line. Thus, the over-expressed BL1 gene conferred enhanced resistance to soft rot in Brassica rapa.
Similar content being viewed by others
Introduction
Brassica rapa (chinese cabbage or ‘Baechu’ in Korea) has been one of the main vegetable crop in Korea, Japan and China for many years due to its economic and nutritional contribution. ‘Seoulbaechu’ is a kind of heading type of Brassica rapa (Baechu) that has an erected compact head and wrinkled leaves with wide veins. Baechu is highly susceptible to bacterial soft rot disease, which is caused by a gram-negative bacterium, Pectobacterium carotovorum subsp. carotovorum (Pcc). Bacterial soft rot is the most severe and destructive disease across members of Brassica. Control of soft rot disease is difficult due to a wide range of hosts, the ability of the bacteria to survive in the plant debris in soil, and host susceptibility. Effective chemical controls are not available so far. Culturing practices, including raised planting beds, reduced plant density, and delayed planting dates can reduce disease frequency and progression, but may reduce agricultural efficiency (Fritz and Honma 1987). Genetic tolerance or resistance may represent an ideal alternative approach. Ren et al. (2001) improved soft rot disease resistance of Baechu through repeated intercrossing. But the traditional recurrent selection procedure is time- and labour-consuming. Moreover, due to lack of natural resistant genes against soft rot disease, the proceeding of routine raising disease-resistance breeding is restricted (Li 1995). Therefore, genetic engineering of soft rot disease tolerance in Baechu is of significant interest in agricultural biotechnology. Recently, chitinase genes showed response to soft rot causing bacterium, Pcc infection in Baechu (Ahmed et al. 2012) and transgenic plants expressing several genes were reported to show resistance to the soft rot-causing bacterial pathogen. Transgenic potato expressing heterogeneously ATP/ADP transporter gene (Linke et al. 2002) and bacterial pheromone N-acyl-homoserine lactone synthesis gene (Toth et al. 2004) showed much improved resistance to soft rot. Transgenic Baechu plants overexpressing bromelain gene (BAA1) was reported to show resistance to bacterial soft rot disease caused by Pcc (Jung et al. 2008).
Cysteine proteases such as papain, bromelain, and ficin are mainly produced by tropical plants and exhibit a number of toxic effects (Mynott et al. 2002). They are involved in signalling pathways and in the response to biotic and abiotic stresses (Grudkowska and Zagdańska 2004). These cysteine proteases have been widely used for various purposes like anti-proliferation (Grabowska et al. 1997), anti-cancer (Garbin et al. 1994), or anti-inflammatory properties (Gaspani et al. 2002). Bromelain is reported to have a number of potential therapeutic applications, including treatment of trauma, inflammation, autoimmune diseases, enhancement of immune response, and malignant disorders (Maurer 2001; Orsini 2006). In addition, Konno et al. (2004) reported that cysteine proteases in latex played an important role in the defence mechanisms of papaya. Bromelain was isolated from the stem of pineapples (Ananas comosus) and was characterized as a complex of cysteine proteases (Batkin et al. 1988; Maurer 2001). When bromelain is used in conjunction with antibiotic therapy, bromelain has been shown to increase antibiotic effectiveness and absorption (Luerti and Vignali 1978; Tinozzi and Venegoni 1978). In addition, bromelain is known to be relatively safe and not to show side effects, toxicity and resistance (Mynott et al. 2002).
The current study investigated the resistance level of the transgenic Baechu plants overexpressing a bromelain gene, BL1against the soft rot causing bacterium, Pectobacterium carotovorum subsp. carotovorum.
Materials and methods
Agrobacterium-mediated transformation to ‘Seoulbaechu’
The BL1 gene was cloned from pineapple (Ananas comosus L. Merrill) and transferred into Lactuca sativa using a binary vector controlled by 35S promoter (35S-BL1) (Jung et al. 2006). Since BL1 and BAA genes are highly homologous to each other and BAA enhanced resistance against soft rot disease (Jung et al. 2008), we used 35S-BL1 vector in this study for transformation into Brassica rapa cv. ‘Seoulbaechu’. The transformation protocol using hypocotyl explants developed for Brassica rapa (Takasaki et al. 1997) was followed in the current study with some modifications. Seeds of Brassica rapa cv. ‘Seoulbaechu’ (Chungwon seed Corp., Korea) were surface-sterilized by washing with 70 % ethanol for 2 min, 1 % sodium hypochlorite for 15 min and double distilled water 3–4 times. Seeds were germinated and grown into 0.1 x MS medium in a culture room maintained at 22–24 °C with a 16 h light/8 h dark photoperiod at a light intensity of 4,500–5,500 lux. Hypocotyls were excised from 6 to 7-day-old seedlings, cut into segments of 2–4 mm in length, and placed onto MS-1 medium and pre-cultured for 24 h under indirect continuous light. Explants were immersed for 30 min at 40 rpm shaking in a suspension of l × l08 bacteria/ml, then returned to feeder plates. After 2 days of co-cultivation with Agrobacterium, explants were transferred to B5-1 medium supplemented with 500 mg/l carbenicillin and kept at 24 °C under continuous light at 7,500 lux intensity for 3–7 days, then transferred to B5-BZ shoot regeneration medium. These explants were cultured for 7 days in B5-BZ medium supplemented with 500 mg/l carbenicillin, 10 mg/l hygromycin, 10 mg/l AgNO3, for 14 days with 500 mg/l carbenicillin, 20 mg/l hygromycin, for 14 days with 500 mg/l carbenicillin, 30 mg/l hygromycin and for 14 days with 500 mg/l carbenicillin, 30 mg/l hygromycin in the same medium. Then the cultures were transferred onto B5-0 shoot maturation medium supplemented with 500 mg/l carbenicillin and 50 mg/l hygromycin. Two weeks later, shoots were trimmed to contain 2–3 nodes and placed on B5 root induction medium supplemented with 2 mg/l IBA, 500 mg/l carbenicillin, 50 mg/l hygromycin. After 2 weeks, the shoots with no roots were re-cut at the base and placed back onto the medium for another 2–4 weeks.
Screening
Total genomic DNA was isolated with the DNeasy Plant Mini Kit (Qiagen, Germany) from transformants. 35S CaMV promoter specific primers (Fw; 5′-TTCAACAAA GGG GTAATATCCGG-3′, Rv; 5′-CGAAGGATAGTGGGATTGTGC-3′) and BL1 gene specific primers (Fw; 5′-ATGGCTTCCAAAGTTCAACTCGTG-3′, Rv; 5′-TCAAGTTTCAGAAACCATCTT-3′) were used to confirm the transformation event. The PCR procedure was started with denaturation at 94 °C for 1 min, annealing at 60 °C for 1 min and extension at 72 °C for 2 min. After 35 amplifying cycles, amplified PCR products were analyzed by electrophoresis.
Selection of transformants and production of T1 and T2 generation
Regenerated transformants (T0) were grown for 45 days at 5 °C for T1 production. Twenty five T1 seeds from each transformants were cultured in pots and grown up to 5~6 leaflets followed by cold treatment to recover T2 seeds. Fifteen to 20 T2 seeds from each T1 lines were placed onto 1/10 strength of MS plate with 50 mg/l hygromycin for antibiotic resistance screening to analyze genetic segregation. After 5 weeks of culture, heterozygote and homozygote events were counted on the basis of hygromycin resistance and susceptibility of plants. Homozygotic T2 lines were selected and harvested from homozygous lines for further experiments.
Transcript of bromelain gene
Real-time quantitative PCR was performed using 1 μl of cDNA in a 25 μl reaction volume using iTaqTM SYBR® Green Super-mix with ROX (California, USA). Specific primers for the gene were used to perform real-time PCR. The thermal cycler conditions were used as follows: 10 min at 95 °C, followed by 40 cycles at 94 °C for 30 s, 58 °C for 30 s, and 72 °C for 45 s. The fluorescent product was detected at the last step of each cycle. Three replications were used for each sample. Amplification, detection, and data analysis were carried out with a Rotor-Gene 6000 real-time rotary analyzer (Corbett Life Science, Australia). Threshold cycle (Ct) represents the number of cycles at which the fluorescence intensity was significantly higher than the background at the initial exponential phase of PCR amplification.
Inoculation of soft rot causing bacteria and evaluation of disease tolerance
In order to examine the relationship between pathogenic bacterial growth and bromelain activity, BL1 gene was cloned into yeast expression vector designated as YEP352 and transformed yeasts were grown in potato dextrose broth (PDB) for 20 h at 28 °C in a shaking incubator. When cells were harvested and the supernatant saved, the cells were homogenized with an ultrasonic dismembrator to break cell membranes. Pcc was inoculated into plain PDB, the supernatant, or homogenized transformed yeast. After inoculation the growth of Pcc was observed at hourly intervals for 12 h using a spectrophotometer. Optical density (OD) was measured at 600 nm for cell growth. The Ren et al. (2001) method was used for plant inoculation. Homozygotic T2 lines and wild type ‘Seoulbaechu’ were directly sown in 50-cell plugs and grown for 3 weeks under greenhouse conditions. When each plant had 4~5 leaflets, a soft rot bacterial solution was inoculated onto the 3rd leaflet with a needle for wound inoculation (WI). These were kept in a mist chamber (100 % relative humidity, 23 °C). In the case of non-wound inoculation (NWI), one month-old seedlings were transplanted in the field with 45 cm × 90 cm spacing. After an adaptation period of two weeks, a bacterial solution was applied onto the 3rd leaflet for inoculation. Inoculated seedlings were mulched and watered three times per day to maintain a high humidity. Three replications were conducted at each experiment. The concentration of bacterial solution was 2.1 × 102 CFU/ml for both experiments. After 10 days of inoculation, disease occurrence rate was evaluated and a rate of infected leaf (RIL) was calculated by dividing the number of infected leaflets by total leaflet per plant ×100 (%).
Results and discussion
Transformation and expression analysis
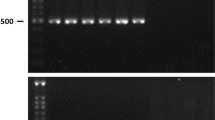
The transgenic plants confirmed by genomic DNA PCR and eight plants were obtained with the BL1 gene insert (Fig. 1a). Among the eight transformant lines, three lines produced transformant and non-transformant in a 3:1 (χ 2 = 0.002–0.03, P = 0.80–0.92) ratio. This pattern of segregation was only possible when a single copy transfer of gene occurs in the transgenic plants. The other transformants were not segregated in such a pattern and this indicates the transfer of more than one copy of the target gene. For that reason, we discarded the other transformants and only BL2-3, BL3-1 and BL8-2 homozygous transformant lines were used for further study. A considerable transcript level of BL1 gene was observed among these homozygous T2 lines in real-time PCR expression analysis (Fig. 1b).
Confirmation of transgenic events and expression analysis of BL1 gene in transgenic chinese cabbage plants. a Insert check in T0 plants using BL1 and 35S CaMV promoter specific primers; b Relative transcription level of BL1 gene in BL2-3, BL3-1 and BL8-2 homozygote lines of T2 generation by real-time PCR. The error bars represent the standard error of the means of three independent replicates
Bacterial growth inhibition by recombinant yeast vector containing BL1 gene
The BL1 gene was expressed in the yeast expression vector designated as YEP352 to investigate whether it can be involved in the inhibition of growth of Pcc or not. The growth of Pcc was dramatically inhibited at an early stage when grown in the media containing homogenized yeast cells harbouring YEP352 (Fig. 2). These homogenized cells contained the most over-expressed BL1 while some were secreted out of the cells. This was an indicator that recombinant bromelain affects pathogen growth at the initial stage of infection.
Growth inhibition of Pectobacterium carotovorum subsp. carotovorum in nutrient broth media. Bacterial growth rate was observed at every hour by spectrophotometer with 600 nm. A; bacterial growth in potato dextrose broth (PDB) as control, B; in externally secreted proteins from YEP352 containing yeasts, C; in internally secreted soluble proteins
Increased soft rot resistance
Soft rot disease occurrence rate was evaluated as % RIL: in BL2-3 and BL3-1 lines, 96 % and 100 % RIL values were observed respectively, which were similar to the wild type (100 % RIL). On the other hand, 75.3 % RIL was observed in the BL8-2 line (Table 1). For the non-wound inoculation (NWI) experiment, 100 % RIL were observed in BL2-3, BL3-1 and wild type at 5 days after NWI. In contrast, BL8-2 showed only 62 % RIL which indicated increased resistance to soft rot disease (Table 2).
Although no information is yet available concerning the mechanism of action of bromelain gene, the observed aggregation of both gram-positive and gram-negative bacteria in vitro might play a role in vivo through the control of pathogen migration to uninfected areas (Segura et al. 1999). Alternatively, this gene might be involved in redox regulation, as it contains putative redox-active cysteines. It is well known that reactive oxygen species (ROS) are involved in pathogenesis and wounding. Recently, it has been shown that GIP2 from Petunia hybrida (GASA-like protein) regulates ROS levels. Consequently, GAST-like proteins containing putative redox-active cysteines may act as antioxidants (Wigoda et al. 2006). Transgenic tobacco and Brassica rapa lines over-expressing Brassica polygalacturonase-inhibiting protein 2 (BrPGIP2) exhibited improved resistance to bacterial soft rot (Hwang et al. 2010). High tolerance to soft rot disease also developed in a transgenic Brassica rapa L. ssp. pekinensis inbred line, Kenshin, by expression of N-acyl-homoserine lactonase (AHL-lactonase) (Vanjildorj et al. 2009).
From these results, we conclude that over-expressed BL1 in homozygote T2 lines of Brassica rapa cv. ‘Seoulbaechu’ could increase resistance to soft rot disease. Although the expression level was varied among the transgenic lines, it was obvious that increased BL1 genetic activity could induce disease tolerance. Further experiments should be performed with other pathogens using BL8-2 homozygotic transgenic lines in order to analyze increased disease tolerance of Brassica rapa.
References
Ahmed, N. U., Park, J. I., Seo, M. S., Kumar, T. S., Lee, I. H., Park, B. S., & Nou, I. S. (2012). Identification and expression analysis of chitinase genes related to biotic stress resistance in Brassica. Molecular Biology Reports, 39, 3649–3657.
Batkin, S., Taussig, S. J., & Szekerezes, J. (1988). Antimetastatic effect of bromelain with or without its proteolytic and anticoagulant activity. Journal of Cancer Research and Clinical Oncology, 114, 507–508.
Fritz, V. A., & Honma, S. (1987). The effect of raised beds, population densities, and planting date on the incidence of bacterial in Chinese cabbage. Journal of the American Society for Horticultural Science, 112, 41–44.
Garbin, F., Harrach, T., Eckert, K., & Maurer, H. R. (1994). Bromelain proteinase F9 augments human lymphocyte-mediated growth inhibition of various tumor cells in vitro. International Journal of Oncology, 5, 197–203.
Gaspani, L., Limiroli, E., Ferrario, P., & Bianchi, M. (2002). In vivo and in vitro effects of bromelain on PGE2 and SP concentrations in the inflammatory exudate in rats. Pharmacology, 65, 83–86.
Grabowska, E., Eckert, K., Fichtner, I., Schulze-Foster, K., & Maurer, H. R. (1997). Bromelain proteases suppresses growth, invasion and lung metastasis of B16F10 mouse melanoma cells. International Journal of Oncology, 11, 243–248.
Grudkowska, M., & Zagdańska, B. (2004). Multifunctional role of plant cysteine proteinases. Acta Biochemica Polonica, 51(3), 609–624.
Hwang, B. H., Bae, H., Lim, H. S., Kim, K. B., Kim, S. J., Im, M. H., Park, B. S., Kim, D. S., & Kim, J. (2010). Overexpression of polygalacturonase-inhibiting protein 2 (PGIP2) of Chinese cabbage (Brassica rapa ssp. pekinensis) increased resistance to the bacterial pathogen Pectobacterium carotovorum ssp. carotovorum. Plant Cell Tissue and Organ Culture, 103, 293–305.
Jung, Y. J., Hyun, K. G., Choi, J. S., Lee, S. Y., Nou, I. S., Park, J. H., & Kang, K. K. (2006). Characterization of transgenic Lettuce (Lactuca sativa L.) using a BL1 gene encoding bromelain isolated from pineapple. Korean Journal of Plant Biotechnology, 33(1), 27–32.
Jung, Y. J., Choi, C. S., Park, J. H., Kang, H. W., Choi, J. E., Nou, I. S., Lee, S. Y., & Kang, K. K. (2008). Overexpression of the pineapple fruit bromelain gene (BAA) in transgenic Chinese cabbage (Brassica rapa) results in enhanced resistance to bacterial soft rot. Electronic Journal of Biotechnology, 11(1).
Konno, K., Hirayama, C., Nakamura, M., Tateishi, K., Tamura, Y., Hattori, M., & Kohno, K. (2004). Papain protects papaya trees from herbivorous insects: role of cysteine proteases in latex. The Plant Journal, 37, 370–378.
Li, S. D. (1995). Progress in disease resistant breed of main vegetables (pp. 96–100). Beijing: Science Press.
Linke, C., Conrath, U., Jeblick, W., Betsche, T., Mahn, A., Düring, K., & Neuhaus, H. E. (2002). Inhibition of the plastidic ATP/ADP transporter protein primes potato tubers for augmented elicitation of defense responses and enhances their resistance against Erwinia carotovora. Plant Physiology, 129(4), 1607–1615.
Luerti, M., & Vignali, M. L. (1978). Influence of bromelain on penetration of antibiotics in uterus, salpinx and ovary. Drugs under Experimental and Clinical Research, 4(1), 45–48.
Maurer, H. R. (2001). Bromelain: biochemistry, pharmacology and medical use. Cellular and Molecular Life Sciences, 58(9), 1234–1245.
Mynott, T. L., Crossett, B., & Prathalingam, S. R. (2002). Proteolytic inhibition of Salmonella enterica serovar typhimurium-induced activation of the mitogen-activated protein kinases ERK and JNK in cultured human intestinal cells. Infection and Immunity, 70(1), 86–95.
Orsini, R. A. (2006). Bromelain. Plastic and Reconstructive Surgery, 118(7), 1640–1644.
Ren, J., Petzoldt, R., & Dickson, M. H. (2001). Genetics and population improvement of resistance to bacterial soft rot in Chinese cabbage. Euphytica, 117, 197–207.
Segura, A., Moreno, M., Madueno, F., Molina, A., & Garcia-Olmedo, F. (1999). Snakin-1, a peptide from potato that is active against plant pathogens. Molecular Plant-Microbe Interactions, 12, 16–23.
Takasaki, T., Hatakeyama, K., Ojima, K., Watanabe, M., Toriyama, K., & Hinata, K. (1997). Factors influencing Agrobacterium-mediated transformation of Brassica rapa L. Breeding Science, 47, 127–134.
Tinozzi, S., & Venegoni, A. (1978). Effect of bromelain on serum and tissue levels of amoxycillin. Drugs under Experimental and Clinical Research, 4, 39–44.
Toth, I. K., Newton, J. A., Hyman, L. J., Lees, A. K., Daykin, M., Ortori, C., Williams, P., & Fray, R. G. (2004). Potato plants genetically modified to produce N-acylhomoserine lactones increase susceptibility to soft rot erwiniae. Molecular Plant-Microbe Interactions, 17(8), 880–887.
Vanjildorj, E., Song, S. Y., Yang, Z. H., Choi, J. E., Noh, Y. S., Park, S., Lim, W. J., Cho, K. M., Han Dae Yun, H. D., & Lim, Y. P. (2009). Enhancement of tolerance to soft rot disease in the transgenic Chinese cabbage (Brassica rapa L. ssp. pekinensis) inbred line, Kenshin. Plant Cell Reports, 28, 1581–1591.
Wigoda, N., Ben-Nissan, G., Granot, D., Schwartz, A., & Weiss, D. (2006). The gibberellin-induced, cysteine-rich protein GIP2 from Petunia hybrida exhibits in planta antioxidant activity. The Plant Journal, 48, 796–805.
Acknowledgements
This research was supported by iPET (Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries), Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Young-Jin Koh and Jong-In Park equally contributed to this work.
Rights and permissions
About this article
Cite this article
Koh, YJ., Park, JI., Ahmed, N.U. et al. Enhancement of resistance to soft rot (Pectobacterium carotovorum subsp. carotovorum) in transgenic Brassica rapa . Eur J Plant Pathol 136, 317–322 (2013). https://doi.org/10.1007/s10658-012-0165-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-012-0165-4