Abstract
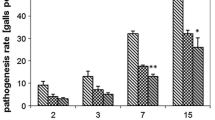
Sustainable strategies for plant-parasitic nematode control are required to reduce dependence on chemical nematicides. Foliar application of various compounds can induce a systemic defence response that reduces nematode infestation. The effects of benzothiadiazole (BTH), β-aminobutyric acid (BABA), jasmonates (cis-jasmone and methyl jasmonate) and salicylic acid (SA) in the development and reproduction of the root-knot nematode Meloidogyne chitwoodi in tomato plants were assessed. The effects of BTH and of the jasmonates were further tested on potato plants. Pot assays were conducted using tomato plants cv. Tiny Tim or potato cv. Désirée treated with foliar sprays and inoculated with 300 second stage juveniles. Nematode development and reproduction were assessed 21 and 45 days after inoculation. Treatment with SA had a negative effect on nematode development in tomato plants but did not affect reproduction and methyl jasmonate treatment was the most effective in reducing nematode penetration (58 %). Nematode development was significantly affected in potato plants sprayed with cis-jasmone. Nematode penetration was reduced by 90, 67 and 81 % in plants treated with BTH, cis-jasmone and methyl jasmonate respectively, although the reproduction factor (Rf) was only significantly lower in the BTH treatment (Rf = 7.6) when compared to the control (Rf = 18.1). Our results suggest that both the SA and JA pathways play an important role in plant defence mechanisms against root-knot nematode development and reproduction for both plants, and should be considered in the design of integrated pest management approaches.




Similar content being viewed by others
References
Berry, S. D., Rutherford, R. S., & Curtis, R. H. (2011). Preliminary investigations into inducing resistance in sugarcane against Meloidogyne incognita. South African Journal of Plant and Soil, 28, 272. abstract.
Byrd, D. W., Kirkpatrick, T., & Barker, K. R. (1983). An improved technique for clearing and staining plant tissues for detection of nematodes. Journal of Nematology, 15, 142–143.
Chinnasri, B., Sipes, B. S., & Schmitt, D. P. (2006). Effects of inducers of systemic acquired resistance on reproduction of Meloidogyne javanica and Rotylenchulus reniformis in pineapple. Journal of Nematology, 38, 319–325.
Cohen, Y. R. (2002). β-aminobutyric acid-induced resistance against plant pathogens. Plant Disease, 86, 448–457.
Collins, H. P., Navare, D. A., Riga, E., & Pierce, F. J. (2006). Effect of foliar applied plant elicitors on microbial and nematode populations in the root zone of potato. Communications in Soil Science and Plant Analysis, 37, 1747–1759.
Cooper, W. R., Jia, L., & Goggin, L. (2005). Effetcs of jasmonate-induced defenses on root-knot nematode infection of resistant and susceptible tomato cultivars. Journal of Chemical Ecology, 31, 1953–1967.
Curtis, R. H., Birkett, M., Pye, B., Pickett, J., & Kerry, B. (2009). Aspects of the plant-nematode interactions: host recognition and plant signalling molecules. 2nd International Congress of Nematology (40th ONTA and 28th SBN Meetings), Maceió, Brasil. [abstract] http://abis.upc.es/onta/sites/default/files/S32-1.
da Conceição, I. L. P. M., Cunha, M. J. M., Feio, G., Correia, M., Vieira dos Santos, C., de O. Abrantes, I. M., et al. (2009). Root-knot nematodes, Meloidogyne spp., on potato in Portugal. Nematology, 11, 311–313.
Eisenback, J. D., & Hirschmann Triantaphyllou, H. (1991). Root-knot nematodes: Meloidogyne species and races. In W. R. Nickle (Ed.), Manual of agricultural nematology (pp. 191–274). New York: Marcel Dekker.
Ferris, H., Carlson, H. L., Viglierchio, D. R., Westerdahl, B. B., Wu, F. W., Anderson, C. E., et al. (1993). Host status of selected crops to Meloidogyne chitwoodi. Supplement to Journal of Nematology, 25, 849–857.
Fujimoto, T., Tomitaka, Y., Abe, Y., Tsuda, S., Futai, K., & Mizukubo, T. (2011). Expression profile of jasmonic acid-induced genes and the induced resistance against the root-knot nematode (Meloidogyne incognita) in tomato plants (Solanum lycopersicum) after foliar treatment with methyl jasmonate. Journal of Plant Physiology, 168, 1084–1097.
Golden, A. M., O’Bannon, J. H., Santo, G. S., & Finley, A. M. (1980). Description and SEM observations of Meloidogyne chitwoodi n. sp. (Meloidogynidae), a root-knot nematode on potato in the Pacific Northwest. Journal of Nematology, 12, 319–327.
Gozzo, F. (2003). Systemic acquired resistance in crop protection: from nature to a chemical approach. Journal of Agricultural and Food Chemistry, 51, 4487–4503.
Gutjahr, C., & Paszkowski, U. (2009). Weights in balance: jasmonic acid and salicylic acid signalling in root-biotroph interactions. Molecular Plant-Microbe Interactions, 7, 763–772.
Heil, M., & Baldwin, I. T. (2002). Fitness costs of induced resistance: emerging experimental support for a slippery concept. Trends in Plant Science, 7, 61–67.
Heil, M., & Bostock, R. M. (2002). Induced Systemic Resistance (ISR) against pathogens in the context of induced plant defences. Annals of Botany, 89, 503–512.
Hussey, R. S., & Barker, K. R. (1973). A comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Disease Reporter, 57, 1025–1028.
Kuć, J. (2001). Concepts and direction of induced systemic resistance in plants and its application. European Journal of Plant Pathology, 107, 7–12.
Lucas, J. (2010). Advances in plant disease and pest management. Journal of Agricultural Science, 149, 91–114.
Molinari, S., & Baser, N. (2010). Induction of resistance to root-knot nematodes by SAR elicitors in tomato. Crop Protection, 29, 1354–1362.
Nandi, B., Kundu, K., Banerjee, N., & Shina Babu, S. P. (2003). Salicylic acid-induced suppression of Meloidogyne incognita infestation of okra and cowpea. Nematology, 5, 747–752.
O’Bannon, J. H., Santo, G. S., & Nyczepir, A. P. (1982). Host range of the Columbian root-knot nematode. Plant Disease, 66, 1045–1048.
OEPP/EPPO. (2009). Meloidogyne chitwoodi and Meloidogyne fallax. EPPO Bulletin, 39, 5–17.
Oka, Y., & Cohen, Y. (2001). Induced resistance to cyst and root-knot nematodes in cereals by DL-β-amino-n-butyric acid. European Journal of Plant Pathology, 107, 219–227.
Oka, Y., Cohen, Y., & Spiegel, Y. (1999). Local and systemic induced resistance to the root-knot nematode in tomato by DL-β-amino-n-butyric acid. Phytopathology, 89, 1138–1143.
Santo, G. S., & O’Bannon, J. H. (1982). Reaction of tomato cultivars to Meloidogyne chitwoodi and M. hapla. Plant Disease, 66, 406–407.
Santo, G. S., Finley, A. M., & Golden, A. M. (1980). Occurrence and host range of a new root-knot nematode (Meloidogyne chitwoodi) in the Pacific Northwest. Plant Disease, 64, 951–952.
Smant, G., & Jones, J. T. (2011). Suppression of plant defences by nematodes. In J. T. Jones, G. Gheysen, & C. Fenoll (Eds.), Genomics and molecular genetics of plant-nematode interactions (pp. 273–286). London: Springer Academic Publishers.
Trudgill, D. L., & Blok, V. C. (2001). Apomitic, polyphagous root-knot nematodes: exceptionally successful and damaging biotrophic root pathogens. Annual Review of Phytopathology, 39, 53–77.
Vallad, G. E., & Goodman, R. M. (2004). Systemic acquired resistance and induced systemic resistance in conventional agriculture. Crop Science, 44, 1920–1934.
Walling, L. L. (2001). Induced resistance: from the basic to the applied. Trends in Plant Science, 6, 445–447.
Acknowledgments
The first author was supported by ESF funds through the “Programa Operacional Potencial Humano - POPH” of the “The National Strategic Reference Framework” (SFRH/BD/27317/2006). Rothamsted Research receives grant aided support from the Biotechnology and Biological Sciences Research Council of the United Kingdom.
We thank Barry Pye for spraying the tomato plants using the Rothamsted Research Experimental Station automatic hydraulic sprayer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vieira dos Santos, M.C., Curtis, R.H.C. & Abrantes, I. Effect of plant elicitors on the reproduction of the root-knot nematode Meloidogyne chitwoodi on susceptible hosts. Eur J Plant Pathol 136, 193–202 (2013). https://doi.org/10.1007/s10658-012-0155-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-012-0155-6