Abstract
In the general population, the lowest mortality risk is considered to be for the body mass index (BMI) range of 20–24.9 kg/m2. In chronic diseases (chronic kidney disease, chronic heart failure or chronic obstructive pulmonary disease) the best survival is observed in overweight or obese patients. Recently above-mentioned phenomenon, called obesity paradox, has been described in patients with coronary artery disease. Our aim was to analyze the relationship between BMI and total mortality in patients after acute coronary syndrome (ACS) in the context of obesity paradox. We searched scientific databases for studies describing relation in body mass index with mortality in patients with ACS. The study selection process was performed according to PRISMA statement. Crude mortality rates, odds ratio or risk ratio for all-cause mortality were extracted from articles and included into meta-analysis. 26 studies and 218,532 patients with ACS were included into meta-analysis. The highest risk of mortality was found in Low BMI patients—RR 1.47 (95 % CI 1.24–1.74). Overweight, obese and severely obese patients had lower mortality compared with those with normal BMI–RR 0.70 (95 % CI 0.64–0.76), RR 0.60, (95 % CI 0.53–0.68) and RR 0.70 (95 % CI 0.58–0.86), respectively. The obesity paradox in patients with ACS has been confirmed. Although it seems to be clear and quite obvious, outcomes should be interpreted with caution. It is remarkable that obese patients had more often diabetes mellitus and/or hypertension, but they were younger and had less bleeding complications, which could have influence on their survival.
Similar content being viewed by others
Background
The concept of obesity (from the Latin word obdere—to eat all over: ob—over, above; edere—to eat) for the first time was used in the Oxford Dictionary in 1611, as a synonym for words: corpulent, thick [1]. The oldest trace of obesity is believed to be a female Willendorf statuette, dated about 22,000–24,000 years B.C. [2].
The attitude toward obesity has been changing over centuries and cultures. In ancient Greece (Hippocrates) and India (Sushruta), it was considered as a pathology [3]. In the Europe and the Far East, in the Middle Ages and the Renaissance, obesity was attractive and desirable. A corpulent silhouette was identified with wealth. In the twentieth and twenty-first century, obesity again became unpopular and unfashionable. Being slim has been considered as optimal weight status both for aesthetic and health reasons.
There are many parameters describing body weight status. Years of observation revealed that body mass and height were in certain proportions. Epidemiological significance of the same body weight is completely different in tall and short person. The most popular formula describing weight in relation to height is the Quetelet index, also known as Body Mass Index (BMI) [4]. BMI is expressed as the ratio of body weight in kilograms and the square of the height in meters. Based on epidemiological observations linking various aspects of health status with BMI, the World Health Organization (WHO) has established a normal BMI for European and North American populations in the range of 18.5–24.9 kg/m2 [5]. A BMI range of 25–29.9 kg/m2 defines overweight and a BMI of 30 kg/m2 and more is regarded as obesity. BMI below 18.5 kg/m2 indicates underweight.
In some populations, the BMI cut-off values for a diagnosis of obesity are different. For example, in the Japanese, South Korean and Chinese populations obesity is recognized for BMIs above 25 kg/m2 [6], 27.5 kg/m2 [7] and 28 kg/m2 [8], respectively.
BMI can be calculated easily and quickly and thus it is widely used both in research and clinical areas. It is also applied for body weight classification by WHO. It should be noted that BMI is not the only and probably not the most accurate measure of the cardiovascular risk associated with body weight.
The obesity, described as higher BMI, is considered as the risk factor for mortality in the general population. The lowest mortality is observed for the BMI range of 20–24.9 kg/m2 (for non-smokers in the American and European populations) and it increases below and above this range [5, 9]. During the last two decades, reports on the favorable prognosis in chronically ill patients with overweight or obesity have been published. This phenomenon commonly called the obesity paradox or reversed epidemiology was recognized in patients with chronic kidney disease [10], chronic heart failure [11] and chronic obstructive pulmonary disease [12]. Recently, a similar paradox linking higher BMI with better prognosis was described in coronary artery disease [13, 14]. Due to acute metabolic imbalance during AMI and increased catabolism following AMI [15], the occurrence of obesity paradox after AMI could be different than in stable CAD.
Objectives
Our aim was to analyze the relationship between BMI and total mortality in patients after acute coronary syndrome (ACS).
Methods
Study design
The meta-analysis were performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [16].
Data sources
PubMed, ScienceDirect and Cochrane Library databases were systematically searched for studies which reported total mortality rates in relation to BMI in patients with acute coronary syndrome. Multiple queries using following keywords were performed on August 27, 2014: (‘body mass index’ OR BMI OR ‘body weight’ OR obesity OR overweight OR underweight) AND (‘acute coronary syndrome’ OR ‘myocardial infarction’ OR ‘unstable angina’) AND (mortality OR death).
Study eligibility criteria for qualitative and quantitative synthesis
Inclusion and exclusion criteria for qualitative and quantitative analyses were presented in Table 1. Studies fulfilling the eligibility criteria were included into analysis.
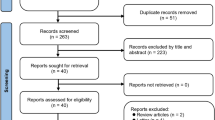
Selection process was shown on Fig. 1 and had been performed according to PRISMA statement [16].
Study appraisal
Studies included in meta-analysis were appraised independently using Newcastle-Ottawa Quality Assessment Scale. Due to restricted inclusion/exclusion criteria, all of the studies had high (at least **) ratings in adequacy of selection and outcomes assessment. Comparability differed between studies, but meta-analysis was conducted on the basis of unadjusted mortality rates (see “Methodology”). Agreement for the quality of the studies was over 90 %.
Data extraction
Two reviewers (J.N. and B.H.) screened independently the titles and abstracts for relevance. Discrepancies between reviewers were discussed until consensus was reached. The articles of selected titles/abstracts were reviewed for inclusion. Using the above-mentioned selection criteria, these 2 reviewers determined independently the articles which were included and excluded. The data from the relevant articles were extracted using predefined extraction forms (Supplemental Appendix Table 1, available online). Any disagreements in data extraction were discussed until consensus was reached.
Methodology
Due to differences in BMI groups between studies in our analysis (see the footnote of Table 2), patients were qualified to the closest BMI group. For the purpose of our meta-analysis subjects were divided into 5 groups: Low BMI, Normal BMI, Overweight, Obesity and Severe obesity. Due to heterogeneity of definitions of underweight used in different studies, in our Low BMI category we included subgroups of patients with BMI below 20 kg/m2. Again, Normal BMI was defined as a BMI range from 18.5 to 25 kg/m2, because in studies various BMI intervals were used i.e. 20–25 or 18.5–24.9 kg/m2 (Table 2). Patients with BMI 25–30 or 30–35 kg/m2 were categorized as Overweight and Obesity, respectively. Severe obesity category comprised patients with BMI ≥ 35 kg/m2. Patients with BMI 35–39.9 kg/m2 and patients with BMI 40 kg/m2 or more were pooled as Severe obese (≥35).
Statistical analyses
A random effects model with inverse variance weighting was used to calculate pooled relative risks (RR) and 95 % confidence interval (CI). Total mortality after ACS was analyzed. Unadjusted mortality rates (2 × 2 or risk ratios) in BMI groups were extracted from studies. Normal BMI group was chosen as the reference one. Heterogeneity between studies was assessed using Cochran Q test and I2 statistic, which denotes the percentage of total variation across studies as a result of heterogeneity rather than chance. All heterogeneity results from analyses of each group were compared with those of the Normal-BMI group. Heterogeneity was considered significant if the P value for the heterogeneity test was less than 0.05. Publication bias was tested by using the Begg and Mazumdar rank correlation test and the Egger’s regression intercept test. In case of significant bias, Duval and Tweedie’s trim and fill method was applied to correct the funnel plot asymmetry. The effect of individual studies was examined by exclusion sensitivity analysis. Each study was removed at a time to assess the degree to which the meta-analysis estimate depends on that particular study.
Results
Study characteristics
Out of the 49 pre-selected articles, 26 met inclusion criteria for meta-analysis [17–42].
218,532 patients with ACS, enrolled in years 1979–2012 were included in the study. Each study contained more men (range between 55.9 and 78.7 %) than women.
Excluded articles with criterion for exclusion were shown in the frame on Fig. 1. To avoid bias due to the differences in diagnostic criteria of overweight and obesity, data from Japanese and South Korean populations were excluded from the analysis (4 studies).
Main analysis
The relative risk ratio for total mortality in patients after ACS with Low BMI was RR 1.74 (CI 1.47–2.05)—Fig. 2. The Begg and Mazumdar rank correlation test was not significant (p = 0.47), but Egger’ s regression intercept test showed significant bias for publications (p = 0.006). The Duval and Tweedie’s Trim and Fill method was used to impute 5 missing studies and estimate RR as 1.47 (1.24–1.74).
Overweight patients had 30 % lower mortality risk after ACS in comparison to those with Normal BMI–RR 0.70 (CI 0.64–0.76)—Fig. 3.
Obesity was related to 40 % lower risk of death after ACS in comparison with Normal-BMI subjects—RR 0.60 (95 % CI 0.53–0.68)—Fig. 4.
Severely obese patients had 30 % lower mortality risk after ACS in comparison to those with Normal BMI—RR 0.70 (CI 0.58–0.86)—Fig. 5.
Both tests used for publication bias assessment were not significant for Overweight, Obesity nor Severe obesity groups.
The relation between risk of mortality and BMI groups was U-shaped—Fig. 6.
Discussion
Age and sex
In 20 of 26 studies, overweight and/or obese patients were younger (1–10 years). Madala et al. [43] observed that the first NSTEMI occurred 12 years earlier in severely obese than in normal BMI patients, whilst only 3.5 years earlier in less endangered overweight group. The finding of younger age of obese patients admitted for ACS therapy could be one of possible explanation for the better survival after ACS in people with BMI ≥ 25 kg/m2. Peto et al. [44] showed that in general population patients with BMIs above 25 kg/m2 had an expected lifetime about 10 years shorter than people with normal BMI. Thus, the percentage of obese people in the population decreases with increasing age.
In patients aged 65 years or older, mortality was higher among obese patients in comparison with those with overweight (p < 0.01) and normal weights (p < 0.001). Obesity in this age group was an independent risk factor for in-hospital mortality [17].
There are different reports on sex distribution across BMI groups. In some studies (Aronson, Eisenstein) more women, while in others [18, 28, 30] more men were included in the obese groups. Rana et al. [19] showed more women in normal-weight and class 1 and 2 obesity with nadir in the overweight ones (39, 33, 40 and 22 %, respectively, p < 0.001). Similar differences were found for cardiogenic shock with occurrence 9.0; 4.1; 3.1; 2.9 and 5.4 % for underweight, normal weight, overweight, class 1 and class 2/3 obesity (p = 0.006), respectively [42].
Comorbidities and complications
Patients with BMI ≥ 25 kg/m2 had higher cardiovascular risk. Diabetes mellitus (20 studies), hypertension (20 studies) or hyperlipidemia (10 studies) were more prevalent in obese than in normal-BMI group. Nevertheless, two studies showed lower GRACE risk score in obese patients [35, 38].
Better survival in overweight or obese patients might be due to the relatively short follow-ups in the studies. During in-hospital stay or even in 5 years after MI, diabetes mellitus or hypertension had little chance to evoke complications and impact the mortality.
Although overweight or obese patients smoked rarely [19–21, 28, 33, 35, 41], mortality risk among current smokers was higher in these groups and rose with increasing BMI–hazard ratio (HR) for BMI > 35 kg/m2 was 4.51 (95 % CI, 1.42–14.3) in comparison to HR 1.18 (95 % CI, 0.42–2.58) for former smokers [19]. Only 8 % of underweight patients smoked in the past in comparison to 15, 16 and 17 % found in normal-weight, overweight and obese subjects respectively (p = 0.001) [21].
Obese patients had higher concentrations of C-reactive protein [27], lower troponin and NT-proBNP levels [45]. The finding of lower natriuretic peptides levels in obese heart failure patients has been recognized recently was and could be explained by clearance function of adipose tissue on these peptides [46].
Compared to normal-BMI group, in obese patients higher estimated glomerular filtration rates by both, MDRD or Cockroft-Gault formulas were observed [25, 36, 47]. The choice of renal function estimation may be important because in patients with coronary artery disease and serum creatinine within normal range, CKD-EPI formula (Chronic Kidney Diseases Epidemiology Initiative) which was derived based on populations with vaster distribution of BMI, predicted long-term outcome more accurately, than MDRD equation [48].
Patients with BMI < 25 had higher risk of bleeding [25, 34]. Nikolsky et al. [25] postulated that the difference had been determined by gastro-intestinal bleeding (2.7 vs 0.4, p = 0.02 for normal weight and obesity, respectively). Moreover, overweight and obese more often had anemia [41] and indication for blood transfusion [25]. Noteworthy, the local groin bleeds (hematoma in the arterial puncture site) occurred also more frequently in patients with normal body weight, compared with overweight and obese (11, 6.8 and 7.6 %, respectively, p = 0.014) [28]. This phenomenon could be explained by ability of fat tissue to compress punctured femoral artery and staunch bleeding.
Obese patients had less often history of stroke [18, 21] and rarely in-hospital stroke [39], but this also could be explained by the differences in age.
Kragelund et al. [21] showed that prevalence of cancer was more likely in underweight women group: 12 vs 5 %, 3 and 4 % in normal-weight, overweight and obese groups respectively (p = 0.001). The observation was confirmed by Angerås et al. [49] (from 8.7 % in underweight to 1.9 % in patients with BMI ≥ 35 kg/m2, p < 0.001).
Diagnosis and treatment
Angiotensin converting enzyme inhibitors (ACEI) were used more frequently in obese as compared to normal weight patients with ACS in 9 studies. Similarly beta-blockers (BB) or statins were given with higher probability to obese patients in 12 and 11 studies respectively. Better pharmacological treatment in obese patients might be caused by existence of other indications for these drugs such as hypertension (20 studies) among obese.
In four studies coronary angiography was reported more often in obese patients [22, 23, 33, 34]. Additionally, six studies reported less frequent percutaneous coronary revascularization in underweight or normal-weight patients with ACS [20, 22, 23, 31, 32, 34].
The door-to-balloon time was significantly longer in obese compared with normal weight patients. Moreover, they had more often final TIMI flow grade 0 compared to normal-weight individuals (2.0 vs. 0.4 %, respectively; p = 0.04) [28]. Initial TIMI flow grade 0 or 1 was also differs between in normal-weight and overweight patients (1.8 vs 0.7 %, respectively, p = 0.04), as well as between overweight and obese subjects (0.7 vs 2.1 %, respectively, p = 0.01) [25].
Multi-vessel coronary artery disease was more common in patients with a normal body weight than in obese with BMI ≥ 40 kg/m2, according to studies of Das et al. (28.4 vs 22.4 %) and Diercks et al. (30.0 vs 24.6 %) [22, 36]. Nikolsky et al. [25] did not confirm the higher occurrence of multi-vessel coronary artery disease in normal-weight with STEMI and showed the same frequency of percutaneous (and surgical) revascularization in all BMI ranges.
Despite the lack of differences in the effect of angioplasty, patients with normal weight required a longer hospital stay: 7.1, 6.9, and 6.7 days for normal weight, overweight, and obese, respectively; p = 0.014. Major adverse cardiovascular events (MACE) at 6 months was also observed more often in the normal BMI range in comparison with overweight and obese cases: 8.8, 6.6, and 5.0 % respectively; p = 0.031 [28]. Major adverse cardiovascular or cerebrovascular events (MACCE) was also more frequent in normal-weight patients, comparing to overweight and obese subjects: 14.7, 12.7, 10.0 %, respectively for in-hospital outcome (p < 0.001) and 12.6, 9.3, 8.7 %, respectively (p < 0.001) for long-term follow-up [31].
Central obesity and weight loss
Only four studies highlighted the prognostic role of central obesity. Zeller et al. divided patients with myocardial infarction (MI) into the tertiles of BMI and waist circumference (WC). The group of lower or middle tertile of BMI and upper tertile of WC had 1-year mortality risk above 20 % in women and more than 18 % in men, whilst in lower WC and upper BMI tertiles mortality was 7.6 and 7.7 %, respectively [50]. This finding was confirmed by Kadakia et al. [45]. It may indicate the special significance of central obesity. Unfortunately, most of the studies did not report parameters allowing more detailed description of obesity phenotype. Kragelund et al. [21] confirmed abdominal obesity assessed by waist-to-hip ratio, to be independent predictor of all-cause mortality in men (adjusted RR 1.22 (1.07–1.38), p < 0.01), but not in women subgroup after ACS [adjusted RR 1.13 (0.95–1.34, p = 0.2)].
Guidelines of European Society of Cardiology (ESC) for the prevention of cardiovascular disease in clinical practice, highlights that obesity in the general population is associated with an increased incidence of cardiovascular disease and cardiovascular mortality. Therefore, the recommendation (class I, level of evidence A) exists for a weight reduction of overweight or obese individuals who have not undergone any cardiovascular event. Body weight reduction to the normal range (BMI 20–24.9 a kg/m2) has a positive effect on blood pressure and plasma lipids, which is reflected in a lower incidence of cardiovascular disease [51]. So far, no studies have confirmed the mortality reduction after MI in patients who reduced their body weight [52]. On the contrary, weight loss of more than 5 % after MI in patients with depression (found in 27 % of patients) was related to 70 % higher risk of all-cause and cardiovascular mortality and those finding were not associated with depression nor social support [29]. Weight loss of more than 5 % in a South Korean population of patients following acute MI was associated with a higher 1-year rate of MACEs. Patients who gained weight also have a greater 1-year mortality risk [7]. On the other hand, intentional weight loss during cardiac rehabilitation in patients with CAD (not MI) was a marker for favourable long-term (6.4 years) outcomes, in both subgroups with initial BMI < 25 or ≥25 kg/m2 [53].
Comparison to general population
The collected data showed that in a population of patients with ACS, an obesity paradox may occur. However, a meta-analysis of 97 studies about mortality in the general population, published in January 2013, indirectly calls into question the existence of the obesity paradox in patients with ACS and chronic diseases. In the general population, the risk of death (HR) in people who were overweight and in the 1st class of obesity (BMI 25–35 kg/m2) was lower than in individuals with normal weights (BMI 18.5–25 kg/m2). Only patients with BMIs 35 kg/m2 and greater had a higher risk of death [54]. To compare the results of the studies about BMI and mortality in chronic diseases with the work of Flegal et al. [54], the obesity paradox exists also in the general population. In the ACS, chronic diseases and the general population the lowest mortality was observed among individuals with BMI values above the normal WHO range.
Although results of our study seem to be clear and quite obvious, outcomes should be interpreted with caution. Despite obese patients more often had diabetes mellitus and/or hypertension, they were younger and had less bleeding complications. Therefore, to compare the mortality of obese patients with people with normal BMIs, the age of the patients and associated diseases should be taken into account in long enough follow-up. In other cases, the relationship between BMI and mortality may be disturbed.
In unadjusted analyses performed on data assessed from the studies, better survival in overweight, obesity and severe obesity group was confirmed in 16 out of 26 studies, 19 of 26 and 5 of 10 studies, respectively. In Low BMI group 7 of 9 studies showed worse survival, comparing to Normal BMI group. After adjustment, both for multivariate analysis (BMI as continuous variable) or models adjusted for various covariables (BMI groups), significant relation between lower BMI and worse survival was found in 15 out of 25 studies.
Conclusion
The existence of obesity paradox in patients with ACS is supported by our meta-analysis.
Limitations
Our study has some limitations and weaknesses.
The analyzed articles varied in methodology. Groups of BMI were categorized using 11 different classification (see footnote of Table 2). Thus, in some studies BMI 19 kg/m2 was classified as ‘Low BMI’, in other—as ‘Normal BMI’. In some publications, underweight patients were excluded from the analyses, because of the ‘extreme high risk of mortality’ [38].
There were lacks of detailed data on race, age, treatment or complications in most of studies, thus those parameters were not shown in the analysis.
The reliability of the data on height and weight is also an important issue. Significant discrepancies between the values measured by physicians and those reported by patients have been shown [54]. Nevertheless, in most ACS cases, weight and height measurements are not possible to conduct, due to life-threatening condition.
References
OED Online [database on the Internet]. Oxford University Press. 2013 [cited November 20, 2013]. http://www.oed.com.
Colman MDE. Obesity in the paleolithic era? The Venus of Willendorf. Endocr Pract. 1998;4(1):58–9.
Tipton CM. Susruta of India, an unrecognized contributor to the history of exercise physiology. J Appl Physiol. 2008;104(6):1553–6. doi:10.1152/japplphysiol.00925.2007.
Gysel C. [Adolphe Quetelet (1796–1874). The statistics and biometry of growth]. Orthod Fr. 1974;45(1):643–77.
Obesity: Preventing and managing the global epidemic: World Health Organization; 2000.
Kanazawa M, Yoshiike N, Osaka T, Numba Y, Zimmet P, Inoue S. Criteria and classification of obesity in Japan and Asia-Oceania. Asia Pac J Clin Nutr. 2002;11:S732–7. doi:10.1046/j.1440-6047.11.s8.19.x.
Kang WY, Hwang SH, Hwang SH, et al. Effects of weight change on clinical outcomes in overweight and obese patients with acute myocardial infarction who underwent successful percutaneous coronary intervention. Chonnam Med J. 2012;48(1):32–8.
Bei-Fan Z, the Cooperative Meta-analysis Group of Working Group on Obesity in C. Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults: study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Asia Pac J Clin Nutr. 2002;11:S685–S93. doi:10.1046/j.1440-6047.11.s8.9.x.
Berrington de Gonzalez A, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363(23):2211–9. doi:10.1056/NEJMoa1000367.
Kalantar-Zadeh K, Streja E, Kovesdy CP, et al. The obesity paradox and mortality associated with surrogates of body size and muscle mass in patients receiving hemodialysis. Mayo Clin Proc. 2010;85(11):991–1001. doi:10.4065/mcp.2010.0336.
Curtis JP, Selter JG, Wang Y. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med. 2005;165(1):55–61. doi:10.1001/archinte.165.1.55.
Landbo C, Prescott E, Lange P, Vestbo J, Almdal T. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(6):1856–61.
Oreopoulos A, Padwal R, Norris CM, Mullen JC, Pretorius V, Kalantar-Zadeh K. Effect of obesity on short- and long-term mortality postcoronary revascularization: a meta-analysis. Obesity. 2008;16(2):442–50. doi:10.1038/oby.2007.36.
Romero-Corral A, Montori VM, Somers VK, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368(9536):666–78. doi:10.1016/S0140-6736(06)69251-9.
Altschule MD, Rosenfeld FM. Increased catabolism following acute myocardial infarction. Arch Intern Med. 1947;80(1):74–80. doi:10.1001/archinte.1947.00220130082007.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12.
Hoit BD, Gilpin EA, Maisel AA, Henning H, Carlisle J, Ross J Jr. Influence of obesity on morbidity and mortality after acute myocardial infarction. Am Heart J. 1987;114(6):1334–41. doi:10.1016/0002-8703(87)90534-5.
Lopez-Jimenez F, Jacobsen SJ, Reeder GS, Weston SA, Meverden RA, Roger VL. PRevalence and secular trends of excess body weight and impact on outcomes after myocardial infarction in the community*. Chest. 2004;125(4):1205–12. doi:10.1378/chest.125.4.1205.
Rana JS, Mukamal KJ, Morgan JP, Muller JE, Mittleman MA. Obesity and the risk of death after acute myocardial infarction. Am Heart J. 2004;147(5):841–6.
Eisenstein EL, McGuire DK, Bhapkar MV, et al. Elevated body mass index and intermediate-term clinical outcomes after acute coronary syndromes. Am J Med. 2005;118(9):981–90.
Kragelund C, Hassager C, Hildebrandt P, Torp-Pedersen C, Kober L. Impact of obesity on long-term prognosis following acute myocardial infarction. Int J Cardiol. 2005;98(1):123–31. doi:10.1016/j.ijcard.2004.03.042.
Diercks DB, Roe MT, Mulgund J, et al. The obesity paradox in non-ST-segment elevation acute coronary syndromes: results from the can rapid risk stratification of unstable angina patients suppress ADverse outcomes with Early implementation of the American College of Cardiology/American Heart Association Guidelines Quality Improvement Initiative. Am Heart J. 2006;152(1):140–8.
Goldberg RJ, Cui J, Olendzki B, et al. Excess body weight, clinical profile, management practices, and hospital prognosis in men and women after acute myocardial infarction. Am Heart J. 2006;151(6):1297–304.
Iakobishvili Z, Danicek V, Porter A, Assali AR, Battler A, Hasdai D. Is increased body mass index associated with a cardioprotective effect after ST-segment-elevation myocardial infarction? Acute Card Care. 2006;8(2):95–8. doi:10.1080/17482940600768673.
Nikolsky E, Stone GW, Grines CL, et al. Impact of body mass index on outcomes after primary angioplasty in acute myocardial infarction. Am Heart J. 2006;151(1):168–75. doi:10.1016/j.ahj.2005.03.024.
Wells B, Gentry M, Ruiz-Arango A, Dias J, Landolfo CK. Relation between body mass index and clinical outcome in acute myocardial infarction. Am J Cardiol. 2006;98(4):474–7. doi:10.1016/j.amjcard.2006.02.053.
Buettner HJ, Mueller C, Gick M, et al. The impact of obesity on mortality in UA/non-ST-segment elevation myocardial infarction. Eur Heart J. 2007;28(14):1694–701. doi:10.1093/eurheartj/ehm220.
Mehta L, Devlin W, McCullough PA, et al. Impact of body mass index on outcomes after percutaneous coronary intervention in patients with acute myocardial infarction. Am J Cardiol. 2007;99(7):906–10. doi:10.1016/j.amjcard.2006.11.038.
Lopez-Jimenez F, Wu CO, Tian X, et al. weight change after myocardial infarction—the enhancing recovery in coronary heart disease patients (ENRICHD) experience. Am Heart J. 2008;155(3):478–84. doi:10.1016/j.ahj.2007.10.026.
Mehta RH, Gitt AK, Jünger C, et al. Body mass index and effectiveness of reperfusion strategies: implications for the management of patients with ST-elevation myocardial infarction. J Interv Cardiol. 2008;21(1):8–14. doi:10.1111/j.1540-8183.2007.00311.x.
Wienbergen H, Gitt A, Juenger C, et al. Impact of the body mass index on occurrence and outcome of acute ST-elevation myocardial infarction. Clin Res Cardiol. 2008;97(2):83–8. doi:10.1007/s00392-007-0585-x.
Aronson D, Nassar M, Goldberg T, Kapeliovich M, Hammerman H, Azzam ZS. The impact of body mass index on clinical outcomes after acute myocardial infarction. Int J Cardiol. 2010;145(3):476–80. doi:10.1016/j.ijcard.2009.12.029.
Hadi HAR, Zubaid M, AlMahmeed W, et al. The prevalence and outcome of excess body weight among middle eastern patients presenting with acute coronary syndrome. Angiology. 2010;61(5):456–64. doi:10.1177/0003319709355801.
Mahaffey KW, Tonev ST, Spinler SA, et al. Obesity in patients with non-ST-segment elevation acute coronary syndromes: Results from the SYNERGY trial. Int J Cardiol. 2010;139(2):123–33.
Shechter M, Hammerman H, Boyko V, Hod H, Behar S, Matetzky S. The obesity paradox in hospitalized acute coronary syndrome patients in Israel: a national survey. CVD Prev Control. 2010;5(3):81–7. doi:10.1016/j.cvdpc.2010.08.002.
Das SR, Alexander KP, Chen AY, et al. Impact of body weight and extreme obesity on the presentation, treatment, and in-hospital outcomes of 50,149 patients with ST-segment elevation myocardial infarction: results from the NCDR (National Cardiovascular Data Registry). J Am Coll Cardiol. 2011;58(25):2642–50. doi:10.1016/j.jacc.2011.09.030.
Timóteo AT, Ramos R, Toste A, Oliveira JA, Ferreira ML, Ferreira RC. Impact of body mass index in the results after primary angioplasty in patients with ST segment elevation acute myocardial infarction. Acute Card Care. 2011;13(3):123–8. doi:10.3109/17482941.2011.606469.
Bucholz EM, Rathore SS, Reid KJ, et al. Body mass index and mortality in acute myocardial infarction patients. Am J Med. 2012;125(8):796–803.
Camprubi M, Cabrera S, Sans J, et al. Body mass index and hospital mortality in patients with acute coronary syndrome receiving care in a university hospital. J Obes. 2012;2012:5. doi:10.1155/2012/287939.
Lazzeri C, Valente S, Chiostri M, et al. Impact of age on the prognostic value of body mass index in ST-Elevation myocardial infarction. Nutrition, metabolism, and cardiovascular diseases: NMCD. 2012.
Herrmann J, Gersh BJ, Goldfinger JZ, et al. Body mass index and acute and long-term outcomes after acute myocardial infarction (from the harmonizing outcomes with revascularization and stents in acute myocardial infarction trial). Am J Cardiol. 2014;114(1):9–16.
Witassek F, Schwenkglenks M, Erne P, Radovanovic D. Impact of Body Mass Index on mortality in Swiss hospital patients with ST-elevation myocardial infarction: does an obesity paradox exist? Swiss Med Wkly. 2014;144:w13986. doi:10.4414/smw.2014.13986.
Madala MC, Franklin BA, Chen AY, et al. Obesity and age of first non-st-segment elevation myocardial infarction. J Am Coll Cardiol. 2008;52(12):979–85. doi:10.1016/j.jacc.2008.04.067.
Peto R, Whitlock G, Jha P. Effects of obesity and smoking on US life expectancy. N Engl J Med. 2010;362(9):855–7. doi:10.1056/NEJMc1000079.
Kadakia MB, Fox CS, Scirica BM, Murphy SA, Bonaca MP, Morrow DA. Central obesity and cardiovascular outcomes in patients with acute coronary syndrome: observations from the MERLIN-TIMI 36 trial. Heart. 2011;97(21):1782–7. doi:10.1136/heartjnl-2011-300231.
Clerico A, Giannoni A, Vittorini S, Emdin M. The paradox of low BNP levels in obesity. Heart Fail Rev. 2012;17(1):81–96. doi:10.1007/s10741-011-9249-z.
Abdulla J, Køber L, Abildstrøm SZ, Christensen E, James WPT, Torp-Pedersen C. Impact of obesity as a mortality predictor in high-risk patients with myocardial infarction or chronic heart failure: a pooled analysis of five registries. Eur Heart J. 2008;29(5):594–601. doi:10.1093/eurheartj/ehn010.
Osadnik T, Wasilewski J, Lekston A, et al. Comparison of modification of diet in renal disease and chronic kidney disease epidemiology collaboration formulas in predicting long-term outcomes in patients undergoing stent implantation due to stable coronary artery disease. Clin Res Cardiol. 2014;103(7):569–76. doi:10.1007/s00392-014-0687-1.
Angerås O, Albertsson P, Karason K, et al. Evidence for obesity paradox in patients with acute coronary syndromes: a report from the Swedish Coronary Angiography and Angioplasty Registry. Eur Heart J. 2013;34(5):345–53. doi:10.1093/eurheartj/ehs217.
Zeller M, Steg PG, Ravisy J, et al. Relation between body mass index, waist circumference, and death after acute myocardial infarction. Circulation. 2008;118(5):482–90. doi:10.1161/circulationaha.107.753483.
Members: ATF, Perk J, De Backer G, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012): the fifth joint task force of the european society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2012;33(13):1635–701. doi:10.1093/eurheartj/ehs092.
Members ATF, Steg PG, James SK, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). Eur Heart J. 2012;. doi:10.1093/eurheartj/ehs215.
Sierra-Johnson J, Romero-Corral A, Somers VK, et al. Prognostic importance of weight loss in patients with coronary heart disease regardless of initial body mass index. Eur J Cardiovasc Prev Rehabil. 2008;15(3):336–40. doi:10.1097/HJR.0b013e3282f48348.
Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309(1):71–82. doi:10.1001/jama.2012.113905.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Niedziela, J., Hudzik, B., Niedziela, N. et al. The obesity paradox in acute coronary syndrome: a meta-analysis. Eur J Epidemiol 29, 801–812 (2014). https://doi.org/10.1007/s10654-014-9961-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-014-9961-9