Abstract
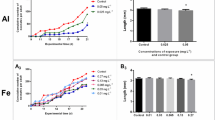
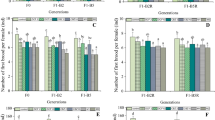
The toxic effects of different concentrations of Triclosan (TCS) (1–128 μg/L) on Daphnia magna (D. magna) were investigated by acute (48 h) and chronic (21-day) toxicity tests. The response of antioxidase system and Phase I metabolism process of D. magna exposed to TCS were investigated by measuring a series of biomarkers including glutathione S-transferase (GST), catalase (CAT), superoxide dismutase (SOD), malondialdehyde (MDA), 7-ethoxyresorufin O-deethylase (EROD), Erythromycin N-demethylase (ERND) and Aminopyrine N-demethylase (APND). The 48 h LC50 of TCS was 330 μg/L for D. magna. In the chronic test, total number of neonates per female, body length and the intrinsic rate of natural increase (r) of D. magna increased at the low exposure concentrations (1–16 μg/L) and decreased at the high concentrations (64–128 μg/L), while the total number of molting per adult decreased continually. The GST and CAT activities showed no significant increase in all treatments, and SOD activities were induced after 24-h exposure and inhibited after 48-h exposure at 4–128 μg/L of concentrations. The MDA content increased after 6-h exposure but decreased after 48-h exposure at 4–128 μg/L. EROD activities initially increased after 6-h exposure, but decreased after 24 and 48-h exposure, ERND and APND activities showed a similar temporal pattern among different treatments groups. SOD, MDA and APND were sensitive to TCS, thus they are suitable as potential biomarkers for the exposure to TCS.


Similar content being viewed by others
References
Aebi H (1974) Catalase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Academic Press, London, pp 671–684
Agarwal A, Gupta S, Sharma RK et al (2005) Role of oxidative stress in female reproduction. Reprod Biol Endocrinol 3(28):1–21
Allen Y, Calow P, Baird DJ (1995) A mechanistic model of contaminant-induced feeding inhibition in Daphnia magna. Environ Toxicol Chem 14(9):1625–1630
Baillieul M, Bervoets L, Blust R, De Boeck G (1993) Assessment of the toxicity of an industrial effluent with a two-generation reproduction test using Daphnia magna. Sci Total Environ 134:1159–1164
Binelli A, Parolini M, Pedriali A, Provini A (2011a) Antioxidant activity in the zebra mussel (Dreissena polymorpha) in response to triclosan exposure. Water Air Soil Poll 217(1–4):421–430
Binelli A, Parolini M, Pedriali A, Provini A (2011b) Antioxidant activity in the zebra mussel (Dreissena polymorpha) in response to triclosan exposure. Water Air Soil Poll 217(1):421–430
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Brown J, Bernot MJ, Bernot RJ (2012) The influence of TCS on the growth and behavior of the freshwater snail, Physa acuta. J Environ Sci Health A 47(11):1626–1630
Calabrese EJ, Baldwin LA (1998) Hormesis as a biological hypothesis. Environ Health Perspect 106(Suppl 1):357
Canesi L, Ciacci C, Lorusso LC, Betti M, Gallo G, Pojana G, Marcomini A (2007) Effects of Triclosan on Mytilus galloprovincialis hemocyte function and digestive gland enzyme activities: possible modes of action on non target organisms. Comp Biochem Physiol C Toxicol Pharmacol 145(3):464–472
Carpenter SR, Cole JJ, Hodgson JR, Kitchell JF, Pace ML, Bade D, Cottingham KL, Essington TE, Houser JN, Schindler DE (2001) Trophic cascades, nutrients, and lake productivity: whole-lake experiments. Ecol Monogr 71(2):163–186
Choi J, Oris JT (2000) Evidence of oxidative stress in bluegill sunfish (Lepomis macrochirus) liver microsomes simultaneously exposed to solar ultraviolet radiation and anthracene. Environ Toxicol Chem 19(7):1795–1799
Ciniglia C, Cascone C, Giudice RL, Pinto G, Pollio A (2005) Application of methods for assessing the geno-and cytotoxicity of Triclosan to C. ehrenbergii. J Hazardous Mat 122(3):227–232
Commission of the European Communities (1996) Technical Guidance Document in Support of Commission Directive 93-67-EEC on Risk Assessment for New Notified Substances and Commission Regulation (EC) No. 1488-94 on Risk Assessment for Existing Substances. Office for official publications of the European communities
Coogan MA, Edziyie RE, La Point TW, Venables BJ (2007) Algal bioaccumulation of triclocarban, triclosan, and methyl-triclosan in a North Texas wastewater treatment plant receiving stream. Chemosphere 67(10):1911–1918
Crapo JD, McCord JM, Fridovich I (1978) Preparation and assay of superoxide dismutases. Methods Enzymol 53:382–393
Dann AB, Hontela A (2011) Triclosan: environmental exposure, toxicity and mechanisms of action. J Appl Toxicol 31(4):285
Daughton CG, Ternes TA (1999) Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ Health Perspect 107(6):907–938
Day K, Kaushik N (1987) An assessment of the chronic toxicity of the synthetic pyrethroid, fenvalerate, to Daphnia galeata mendotae, using life tables. Environ Pollut 44(1):13–26
DeLorenzo ME, Fleming J (2008) Individual and mixture effects of selected pharmaceuticals and personal care products on the marine phytoplankton species Dunaliella tertiolecta. Arch Environ Contam Toxicol 54(2):203–210
EPA US (2002) Methods of measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms. vol EPA-821-R-02-012. Office of Research and Development. US EPA, Washington DC,USA
Fent K, Bucheli TD (1995) Inhibition of hepatic microsomal mono-oxygenase system by organotins in fish. Mar Environ Res 39:351–352
Flaherty CM, Dodson SI (2005) Effects of pharmaceuticals on Daphnia survival, growth, and reproduction. Chemosphere 61(2):200–207
Foran C, Bennett E, Benson W (2000) Developmental evaluation of a potential non-steroidal estrogen: triclosan. Mar Environ Res 50(1):153–156
Gatidou G, Vassalou E, Thomaidis NS (2010) Bioconcentration of selected endocrine disrupting compounds in the Mediterranean mussel, Mytilus galloprovincialis. Mar Pollut Bull 60(11):2111–2116
Hanazato T (1998) Growth analysis of Daphnia early juvenile stages as an alternative method to test the chronic effect of chemicals. Chemosphere 36(8):1903–1909
Ishibashi H, Matsumura N, Hirano M, Matsuoka M, Shiratsuchi H, Ishibashi Y, Takao Y, Arizono K (2004) Effects of triclosan on the early life stages and reproduction of medaka Oryzias latipes and induction of hepatic vitellogenin. Aquat Toxicol 67(2):167–179
Lampert W, Fleckner W, Rai H, Taylor BE (1986) Phytoplankton control by grazing zooplankton: a study on the spring clear-water phase. Limnol Oceanogra 31:478–490
Lemaire P, Förlin L, Livingstone DR (1996) Responses of hepatic biotransformation and antioxidant enzymes to CYP1A-inducers (3-methylcholanthrene,[beta]-naphthoflavone) in sea bass (Dicentrarchus labrax), dab (Limanda limanda) and rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 36(3–4):141–160
Lin DS, Zhou QX, Xie XJ, Liu Y (2010) Potential biochemical and genetic toxicity of triclosan as an emerging pollutant on earthworms (Eisenia fetida). Chemosphere 81(10):1328–1333
Lipnick R (1995) Structure-activity relationships. Fundamentals of aquatic toxicology. Taylor and Francis, Washington, DC, pp 609–655
Livingstone D (2001) Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar Pollut Bull 42(8):656–666
Lopez-Avila V, Hites RA (1980) Organic compounds in an industrial wastewater. Their transport into sediments. Environ Sci Technol 14(11):1382–1390
Lotka AJ A Natural Population Norm I & II. In, 1913. J. Washington. Acad. Science
Matozzo V, Formenti A, Donadello G, Marin MG (2012) A multi-biomarker approach to assess effects of Triclosan in the clam Ruditapes philippinarum. Mar Environ Res 74:40–46
Moss T, Howes D, Williams FM (2000) Percutaneous penetration and dermal metabolism of triclosan (2,4,4′-trichloro-2′-hydroxydiphenyl ether). Food Chem Toxicol 38(4):361–370
Münzinger A, Monicelli F (1992) Heavy metal co-tolerance in a chromium tolerant strain of Daphnia magna. Aquat Toxicol 23(3):203–216
Nash T (1953) The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem J 55(3):416
Nassef M, Matsumoto S, Seki M, Khalil F, Kang IJ, Shimasaki Y, Oshima Y, Honjo T (2010) Acute effects of triclosan, diclofenac and carbamazepine on feeding performance of Japanese medaka fish (Oryzias latipes). Chemosphere 80(9):1095–1100
Newton APN, Cadena SMSC, Rocha MEM, Carnieri EGS, de Oliveira MBM (2005) Effect of triclosan (TRN) on energy-linked functions of rat liver mitochondria. Toxicol Lett 160(1):49–59
OECD (1984) OECD Guidelines for testing of chemicals: Daphnia spp. Acute Immobilisation Test and Reproduction Test. Vol. 202. OECD Publishing, Paris, France. pp:1–16
OECD (1998) OECD Guidelines for Testing of Chemicals: Daphnia magna Reproduction Test. OECD TG 211. In: Annex I: OECD Test Guidelines for Studies Included in the SIDS. Accessed 7 October 2008
Oliveira R, Domingues I, Grisolia CK, Soares AMVM (2009) Effects of triclosan on zebrafish early-life stages and adults. Environ Sci Pollut Res 16(6):679–688
Orvos DR, Versteeg DJ, Inauen J, Capdevielle M, Rothenstein A, Cunningham V (2002) Aquatic toxicity of triclosan. Environ Toxicol Chem 21(7):12
Palenske NM, Nallani GC, Dzialowski EM (2010) Physiological effects and bioconcentration of triclosan on amphibian larvae. Comp Biochem Physiol C Toxicol Pharmacol 152(2):232–240
Peng X, Yu Y, Tang C, Tan J, Huang Q, Wang Z (2008) Occurrence of steroid estrogens, endocrine-disrupting phenols, and acid pharmaceutical residues in urban riverine water of the Pearl River Delta, South China. Sci Total Environ 397(1):158–166
Pohl RJ, Fouts JR (1980) A rapid method for assaying the metabolism of 7-ethoxyresorufin by microsomal subcellular fractions. Anal Biochem 107(1):150–155
Raut SA, Angus RA (2010) Triclosan has endocrine-disrupting effects in male western mosquitofish, Gambusia affinis. Environ Toxicol Chem 29(6):1287–1291
Reiss R, Mackay N, Habig C, Griffin J (2002) An ecological risk assessment for triclosan in lotic systems following discharge from wastewater treatment plants in the United States. Environ Toxicol Chem 21(11):2483–2492
Riva C, Cristoni S, Binelli A (2012) Effects of triclosan in the freshwater mussel Dreissena polymorpha: a proteomic investigation. Aqua Toxicol 118–119:62–71
Rolf U, Paull DH (2005) Co-occurrence of triclocarban and triclosan in US water resources. Environ Sci Technol 39(6):1420–1426
Sanderson H, Brain RA, Johnson DJ, Wilson CJ, Solomon KR (2004) Toxicity classification and evaluation of four pharmaceuticals classes: antibiotics, antineoplastics, cardiovascular, and sex hormones. Toxicology 203(1):27–40
Sarkar A, Ray D, Shrivastava AN, Sarker S (2006) Molecular biomarkers: their significance and application in marine pollution monitoring. Ecotoxicology 15(4):333–340
Singer H, Müller S, Tixier C, Pillonel L (2002) Triclosan: occurrence and fate of a widely used biocide in the aquatic environment: field measurements in wastewater treatment plants, surface waters, and lake sediments. Environ Sci Technol 36(23):4998–5004
Stark JD, Tanigoshi L, Bounfour M, Antonelli A (1997) Reproductive potential: its influence on the susceptibility of a species to pesticides. Ecotoxicol Environ Saf 37(3):273–279
Stebbing A (1982) Hormesis—the stimulation of growth by low levels of inhibitors. Sci Total Environ 22(3):213–234
Tatarazako N, Ishibshi H, Teshima K, Kisbi K, Arizono K (2004) Effects of triclosan on various aquatic organisms. Environ Sci 11(2):133–140
Vaccaro E, Giorgi M, Longo V, Mengozzi G, Gervasi P (2003) Inhibition of cytochrome P450 enzymes by enrofloxacin in the sea bass (Dicentrarchus labrax). Aquat Toxicol 62(1):27–33
Valavanidis A, Vlahogianni T, Dassenakis M, Scoullos M (2006) Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol Environ Saf 64(2):178–189
Van der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmcol 13(2):57–149
van Leeuwen CJ, Luttmer WJ, Griffioen PS (1985) The use of cohorts and populations in chronic toxicity studies with Daphnia magna: a cadmium example. Ecotoxicol Environ Saf 9(1):26–29
Van Leeuwen C, Vermeire T, Vermeire T (2007) Risk assessment of chemicals: an introduction. Springer Verlag
Veldhoen N, Skirrow RC, Osachoff H, Wigmore H, Clapson DJ, Gunderson MP, Van Aggelen G, Helbing CC (2006) The bactericidal agent triclosan modulates thyroid hormone-associated gene expression and disrupts postembryonic anuran development. Aquat Toxicol 80(3):217–227
Villarroel M, Ferrando M, Sancho E, Andreu E (2000) Effects of tetradifon on Daphnia magna during chronic exposure and alterations in the toxicity to generations pre-exposed to the pesticide. Aquat Toxicol 49(1–2):39–47
Wang LQ, Falany CN, James MO (2004) Triclosan as a substrate and inhibitor of 3′-phosphoadenosine 5′-phosphosulfate-sulfotransferase and UDP-glucuronosyl transferase in human liver fractions. Drug Metab Dispos 32(10):1162–1169
Whyte JJ, Jung R (2000) EROD activity. Crit Rev Toxicol 30(4):347–570
Xu W, Zhang G, Zou S, Li X, Liu Y (2007) Determination of selected antibiotics in the Victoria Harbour and the Pearl River, South China using high-performance liquid chromatography-electrospray ionization tandem mass spectrometry. Environ Pollut 145(3):672–679
Yang LH, Ying GG, Su HC, Stauber JL, Adams MS, Binet MT (2008) Growth-inhibiting effects of 12 antibacterial agents and their mixtures on the freshwater microalga Pseudokirchneriella subcapitata. Environ Toxicol Chem 27(5):1201–1208
Zhao JL, Zhang QQ, Chen F, Wang L, Ying GG, Liu YS, Yang B, Zhou LJ, Liu S, Su HC, Zhang RQ (2013) Evaluation of triclosan and triclocarban at river basin scale using monitoring and modeling tools: implications for controlling of urban domestic sewage discharge. Water Res 47:395–405
Acknowledgments
This research was funded by the National Natural Science Foundation of China (U1133005) and the Program of National Science and Technology Development (2012BAC07B05). We also thank to Prof. Vladimir Zitiko from St. Andrews Biology Station, New Brunswick, Canada and Prof. Larry B. Liddle from Long Island University, USA for their helps in language editing of our manuscript.
Conflict of interest
The authors declare there is no conflict of interest for the present manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peng, Y., Luo, Y., Nie, XP. et al. Toxic effects of Triclosan on the detoxification system and breeding of Daphnia magna . Ecotoxicology 22, 1384–1394 (2013). https://doi.org/10.1007/s10646-013-1124-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-013-1124-3