Abstract
Controls on mercury bioaccumulation in lotic ecosystems are not well understood. During 2007–2009, we studied mercury and stable isotope spatial patterns of macroinvertebrates and fishes from two medium-sized (<80 km2) forested basins in contrasting settings. Samples were collected seasonally from multiple sites across the Fishing Brook basin (FBNY), in New York’s Adirondack Mountains, and the McTier Creek basin (MCSC), in South Carolina’s Coastal Plain. Mean methylmercury (MeHg) concentrations within macroinvertebrate feeding groups, and mean total mercury (THg) concentrations within most fish feeding groups were similar between the two regions. However, mean THg concentrations in game fish and forage fish, overall, were much lower in FBNY (1300 and 590 ng/g dw, respectively) than in MCSC (2300 and 780 ng/g dw, respectively), due to lower trophic positions of these groups from FBNY (means 3.3 and 2.7, respectively) than MCSC (means 3.7 and 3.3, respectively). Much larger spatial variation in topography and water chemistry across FBNY contributed to greater spatial variation in biotic Hg and positive correlations with dissolved MeHg and organic carbon in streamwater. Hydrologic transport distance (HTD) was negatively correlated with biotic Hg across FBNY, and was a better predictor than wetland density. The small range of landscape conditions across MCSC resulted in no consistent spatial patterns, and no discernable correspondence with local-scale environmental factors. This study demonstrates the importance of local-scale environmental factors to mercury bioaccumulation in topographically heterogeneous landscapes, and provides evidence that food-chain length can be an important predictor of broad-scale differences in Hg bioaccumulation among streams.
Similar content being viewed by others
Introduction
Mercury (Hg) concentrations commonly exceed human and wildlife health guidelines in forested streams of the eastern United States (Driscoll et al. 2007; Scudder et al. 2009), including many that lack Hg point sources (Fitzgerald et al. 1998; Lindberg et al. 2007). In these ecosystems, atmospheric deposition delivers Hg to the landscape (Hammerschmidt and Fitzgerald 2005) and land cover characteristics, particularly the extent of wetlands, influence its transformation to methylmercury (MeHg) and subsequent delivery of this bioavailable form to streams (St. Louis et al. 1996; Kolka et al. 1999; Wiener et al. 2006; Brigham et al. 2009; Scudder et al. 2009). The contribution of shallow (surface and shallow-subsurface flow systems) geochemical exchange between terrestrial compartments and aquatic habitats in streams makes them particularly sensitive to watershed characteristics that foster MeHg production and transport (Ward et al. 2010). Hg bioaccumulation in streams without Hg point sources has been strongly correlated to forest cover, wetlands extent and connectivity, and hydrologic alteration (St. Louis et al. 1994; Rypel et al. 2008; Scudder et al. 2009; Ward et al. 2010), as well as to aqueous MeHg, dissolved organic carbon (DOC) quantity and aromaticity, and pH (Krabbenhoft et al. 1995; Mason et al. 1995; Driscoll et al. 1998; Grigal 2002; Chasar et al. 2009; Brigham et al. 2009).
Trophic position is an important predictor of MeHg concentrations of consumer organisms within streams (Chasar et al. 2009; Ward et al. 2010), and is an important predictor of Hg in top predator fishes across lakes (Chen and Folt 2005), but has not factored as highly in prediction of fish Hg across streams and rivers (Chasar et al. 2009; Ward et al. 2010). Many stream studies have covered relatively broad geographic, source condition, and (or) hydro-geomorphic extents, which emphasize landscape and associated physiochemical controls [Brumbaugh et al. 2001; Kamman et al. 2005; Chasar et al. 2009; Scudder et al. 2009; U.S. Environmental Protection Agency (USEPA) 2009; Glover et al. 2010]. However, studies addressing a limited range of environmental conditions (spatial coverage, Hg inputs, geochemical characteristics, land cover) are more appropriate for assessing the influence of community structure or trophic relationships on MeHg bioaccumulation (Tsui et al. 2009). These are critical for resource management, yet generally are lacking for small streams (less than 100 km2).
The current study was conducted over 3 years in two medium-sized (<80 km2) basins, Fishing Brook in the central Adirondack Mountain region of NY (FBNY) and McTier Creek in the Sand Hills portion of the inner Coastal Plain of SC (MCSC). Both basins are considered “biological Hg hotspots” (sensu Evers et al. 2007), with (1) relatively high Hg deposition (National Atmospheric Deposition Program Mercury Deposition Network, http://nadp.sws.uiuc.edu/mdn/), (2) substantial forests and riparian wetlands within the watershed, (3) seasonally fluctuating water levels, and (4) resident fish and other biota with Hg concentrations that exceed both human and wildlife fish consumption advisory levels (Evers et al. 2007; Driscoll et al. 2007; Glover et al. 2010; NYDOH 2010; SCDHEC 2010). These basins differ from each other with respect to food web, latitude, topography, trophic diversity, climate, hydrology, wetlands type, and geochemistry. Thus, comparison of within-basin spatial patterns of Hg bioaccumulation between these two contrasting basins should provide insight into important environmental controls on Hg bioaccumulation in small stream basins. The objectives of this study were to (1) describe spatial variation in Hg concentrations in selected lower consumer feeding groups within the FBNY and MCSC basins, (2) describe the relationship of biotic Hg with potential biological, chemical, and landscape controls in each basin, and (3) compare patterns and potential drivers between the two study areas.
Methods
Site selection and basin characterization
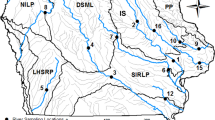
Sites were selected to include a range of landscape and geomorphic characteristics across each study basin (Fig. 1; Table 1). Eight sites were selected within FBNY and four were selected within MCSC. In keeping with its more topographically diverse setting, the topographic and land cover variation among FBNY sites was much greater than that among MCSC sites (Table 1). For example, wetland variation was more than ten times greater across FBNY than across MCSC, and the amount of open water in FBNY ranged more than tenfold (from 0 to more than 10% of basin area), while open water in MCSC was less than 2% of basin area in all cases. The FBNY sites also covered large ranges of landscape slope, drainage density, and distance to stream channel (Online Resource #1). These characteristics are captured by the hydrologic transport distance (HTD) metric, which was derived from the 10-m National Elevation Dataset (http://seamless.usgs.gov, accessed 8 March 2010) as the overland flow distance metric (OFD, System for Automated Geoscientific Analysis; Cimmery 2007). We believe this metric represents shallow subsurface flow paths to the stream network across the FBNY study area. At high flow conditions, some of this flow may occur as overland flow (Schelker et al. 2011), but at the low-to-moderate flow conditions that prevail during much of the growing season, most water moves as shallow subsurface flow. Therefore, we use the term “HTD” in this study to avoid connoting flow over the land surface. FBNY sites covered a much wider range of mean HTD than the MCSC sites (85 vs. 17 m), and included the smallest (146 m) and largest (241 m) mean HTD values among all study sites (both basins).
Locations of Fishing Brook and McTier Creek study areas in the eastern United States, and locations of sites from which biota were collected during 2007–2009 for Hg analysis. Site names are given in Table 1
FBNY in-stream habitat varies among sites, from cascading riffles with limited pool or run habitat (site UT) to valley bottom sinuous channels with alternating pools and runs and limited or no riffle habitat (sites S1, S2, F2). The basin outlet site, F3, differs from all of the other sites in that it is the downstream end of a long, shallow ‘run-of the river’ impoundment, which is a common feature throughout the region. Fish and invertebrate habitat in FBNY consists of woody debris, rocky substrate, and occasional areas of submerged or emergent macrophytes. MCSC stream sites are generally long runs and pools, with mainly sandy substrate, and habitat structure consisting of largely woody debris, leaf packs, undercut banks, deep pools, and rootwads along the banks, with few areas of submerged and emergent macrophytes.
Targeted taxa
Macroinvertebrate and fish taxa targeted for collection were those that represented the breadth of primary and secondary consumer functional feeding groups in the study streams, and that could be collected in sufficient numbers and mass from multiple sites and collection periods. Functional feeding group classifications follow Merritt and Cummins (1996) and Thorp and Covich (2001) for macroinvertebrates, and the regional guides of Smith (1985) and Rohde et al. (1994) for fishes of NY and SC, respectively. For macroinvertebrates, the targeted functional feeding groups were shredders (mainly consume living and/or decomposing plant material), scrapers (mainly consume periphyton and associated biofilm), omnivores (consume both plant and animal material), and predators (mainly consume other macroinvertebrates). Invertebrate families within each of the above feeding groups were targeted as follows: shredders—northern case-maker caddisflies (Trichoptera: Limnephilidae); scrapers—flathead mayflies (Ephemeroptera: Heptageniidae); omnivores—crayfish (Decapoda: Cambaridae); and predators—dragonflies (Odonata: Aeshnidae and Libellulidae; kept separate in processing). Because crayfish and dragonflies can cover a large range of sizes (instars, in the case of dragonflies); these were separated into composites of like-sized individuals in field-processing.
Targeted fish taxa included both “forage fish” and predatory game fish. Forage fish are typically prey for larger predatory fish, and are smaller, shorter-lived species, with varying degrees of herbivory, detritivory, invertivory, or omnivory. The primary targeted forage fishes were invertivore/herbivores, particularly common shiner (Luxilus cornutus) and creek chub (Semotilus atromaculatus) in FBNY, and shiners (Notropis spp., primarily yellowfin shiner, N. lutipinnis) in MCSC. Blacknose dace (Rhinicthys atratulus, an invertivore) and northern redbelly dace (Phoxinus eos, an herbivore/invertivore) were also collected from FBNY, and tessellated darter (Etheostoma olmstedi) and blackbanded darter (Percina nigrofasciata), which are both benthic invertivores, were also collected from MCSC. For predatory game fish, we targeted piscivorous, locally important game species that are widely distributed in small streams of each region. Brook trout (Salvelinus fontinalis) >180 mm total length (TL) was the predatory game fish targeted in FBNY. The brook trout diet, although predominately invertebrates, does include other fish (Smith 1985), and brook trout are typically the top predator in small Adirondack streams. Small streams in the MCSC study area can support a larger variety of predatory game fish (Rohde et al. 1994), so multiple species were included, as follows: largemouth bass (Micropterus salmoides) >200 mm TL, warmouth (Chaenobryttus gulosus) >100 mm TL, chain pickerel (Esox niger) >200 mm TL, redfin pickerel (E. americanus americanus) >150 mm TL, American eel (Anguilla rostrata), and bullhead species (Ameiurus spp.). The full list of macroinvertebrate and fish taxa collected is provided in Online Resource #2.
Sample collection and field processing
Biota and stream water samples were collected seasonally from spring through fall during 2007–2009. Macroinvertebrates were collected from all sites. Fish were collected from a subset of FBNY sites and from all MCSC sites. The basin outlet sites (F3 and M2, Fig. 1) were sampled 8 and 7 times, respectively, during the course of the study; most other sites were sampled 3–5 times. Field measurements of pH and sampling of stream water were conducted within a week of biotic sampling. Water samples were collected with trace-metal clean techniques, and analyzed for filtered-water methylmercury (FMeHg) and DOC as described in Bradley et al. (2011).
Macroinvertebrates were collected by hand-picking, kick-netting, and bank-jabbing from all distinct habitat types (including cobbles, soft surface bed sediment, macrophytes and woody debris) with the goal of collecting three taxon-specific composites of 30 individuals each or at least 1 g wet weight per composite, with a minimum of 15 like-sized individuals per composite. Fish were collected by electrofishing, angling, and passive capture with traps and gill nets.
Specimens were placed in new plastic zip-seal bags with site water, and stored in coolers on wet ice for field processing as soon as possible. The average holding time for all samples was approximately 4 h. Field processing of macroinvertebrates and fish was done in accordance with trace-metal clean techniques (detailed in Scudder et al. 2008). Macroinvertebrates were sorted with pre-cleaned plastic forceps, rinsed in de-ionized water, dried, weighed, and stored on dry ice. Forage fish were processed as whole-body specimens, either individually or as composites of similarly sized individuals. Forage fish were weighed, measured (TL), rinsed in de-ionized water, and double-bagged. Predatory game fish were weighed, measured, and rinsed in de-ionized water. A standard skinless fillet was collected from one side, rinsed, weighed, and double-bagged. All fish samples were immediately placed on dry ice for transport to the laboratory, where they were kept frozen until further processing and analysis.
Hg and stable isotope analysis
Fish tissue was analyzed for total mercury (THg); MeHg (the form of Hg that is accumulated in organisms through diet) is known to comprise >95% of the Hg in fish tissue (Grieb et al. 1990; Bloom 1992). Macroinvertebrates were directly analyzed for MeHg due to the potential for widely varying taxonomic differences in MeHg to THg ratios (Mason et al. 2000). Henceforth, biotic Hg refers to MeHg; either directly measured (macroinvertebrates), or measured as THg and assumed to be primarily MeHg (fish).
Prior to analysis, samples were freeze-dried to constant weight and ground (in their entirety) to a fine powder, with a stainless-steel ball mill (Retsch Model MM200) or an ultracentrifugal mill (Retsch Model ZM200). Macroinvertebrate samples were analyzed for MeHg at the U.S. Geological Survey Wisconsin Hg Research Laboratory, with a dilute nitric acid extraction and cold-vapor atomic fluorescence spectroscopy (Hammerschmidt and Fitzgerald 2005). Laboratory precision for triplicates was 7.6% (±7.2% standard deviation, s.d.), and accuracies for MeHg concentration in blind submissions of standard reference materials, as mean percentage of certified MeHg value ± s.d., were as follows: NIST 2976 (90.9 ± 27.4%); TORT-2 (93.1 ± 14.2%); NRCC DOLT-3 (83.5 ± 9.7%). Fish samples were analyzed for THg at the Trace Element Research Laboratory (Texas A&M University, College Station, Texas) with USEPA Method 7473 (combustion and atomic absorption with a Milestone DMA-80 direct Hg analyzer). Accuracies for THg concentration in blind submissions of standard reference materials, as mean percentage of certified THg value ± s.d., were: NIST 2976 (90.5 ± 14.5%); TORT-2 (118.5 ± 23.5%); and NRCC DOLT-3 (98.2 ± 10.7%).
Macroinvertebrate and fish samples were also analyzed for δ15N and δ13C. These stable isotope analyses were conducted at the Stable Isotope Geochemistry Laboratory at Florida State University’s National High Magnetic Field Laboratory. Samples were analyzed with a ThermoQuest NC2500 Elemental Analyzer interfaced with a Finnegan MAT Delta Plus XP isotope ratio mass spectrometer. Isotope ratios were measured relative to reference gases and calibrated to known carbon and nitrogen standards (δ13CPDB and δ15Nair, ranging from −12.7 to −32.1‰ and −5.3 to 2.5‰, respectively). Additional QA/QC included blind duplicates and blind standard reference material samples (glutamic acid, USGS-40, NIST-8573) included in sample runs (approximately two for every ten samples). Precision and accuracy for nitrogen and carbon isotopic ratios were <0.4‰, and generally <0.2‰, respectively.
Data analysis
Preliminary analysis of stable isotopes was done to verify functional feeding group assignment, to ensure the absence of strong seasonal and (or) annual patterns within sites before pooling samples among seasons and years, and to ensure trophic dependence within sites among prey and consumers. A summary of δ15N and δ13C data used in these analyses is provided in Online Resource #2. Normalization of δ13C to lipid content was not done because corrections are generally small (<1‰) for epibenthic macroinvertebrates and fish, and non-normalized data are appropriate as a screening tool for diet dependence at the ecosystem scale (Post et al. 2007). Data were pooled across seasons and years after preliminary analyses indicated limited, nonsignificant, or inconsistent temporal variation within sites and taxa in Hg concentration and stable isotopes.
A one-way analysis of variance (ANOVA) followed by Tukey’s HSD post-hoc multiple comparison tests was used to examine differences between group means in biotic Hg, δ15N, and estimated trophic position (i.e., taxon- or group-specific regional differences, differences among sites within regions, and differences among feeding groups). Hg concentrations were log-transformed when necessary to meet assumptions of normal distribution before ANOVA, and Welch’s ANOVA was used in cases of failure of Levene’s test for homogeneity of variances. Spearman rank correlation was used to examine the relation of group mean Hg concentrations with environmental variables. A Type I error (α) of 0.05 was used as a significance rule for all statistical tests, unless otherwise noted. Chemical data were pooled from base-flow samples collected multiple times (see Online Resource #3) per season during April through November in 2007–2009, and calculated as a growing season mean over spring, summer, and fall periods. Arithmetic means were used where all values were detected. Occasionally, FMeHg was not detected (<0.04 ng/L); in these cases, maximum likelihood means (Helsel 2005) were used (obtained from LIFEREG procedure in SAS). At site UT, all FMeHg samples were <0.04 ng/L, thus mean FMeHg was <0.04 ng/L. Growing-season means were a simple arithmetic mean of spring, summer, and fall means. Concentrations of FMeHg below the detection limit (0.04 ng/L) were treated as the detection limit value for plotting. Statistical analyses were done in SAS software, version 9.1 (SAS Institute, Inc., Cary, NC).
Spatial comparisons of biotic Hg were evaluated by taxon or feeding group (to minimize variability introduced by differences in feeding ecology), as well as (for regional comparison) by the broader ‘forage fish’ and ‘game fish’ groupings. Analyses of within-basin spatial patterns within each region focused on taxa and feeding groups collected from at least five of the eight FBNY sites or three of the four MCSC sites. Data were pooled across sampling periods after preliminary analysis within sites and taxa indicated limited or no significant seasonal or annual bias in Hg and δ15N. Biotic Hg concentrations were based on dry weight, except where fish Hg was expressed as wet-weight concentration for comparison with human and wildlife consumption guidelines. A moisture content of 80% was applied uniformly for this conversion.
Average trophic position was calculated for consumer groups to account for potential site-to-site differences in δ15N of nitrogen source inputs. Trophic position was calculated by taking the group mean of the difference between sample δ15N and the mean δ15N of the invertebrate feeding group consistently having the lowest δ15N across sites (Anderson and Cabana 2007), and applying a fractionation constant of 3.4 (Post 2002).
Results
Shredders were the most widely collected macroinvertebrate feeding group across FBNY (collected from all eight sites), followed by predators (six sites). Predators and omnivores were the most widely collected across MCSC (four sites), but shredders were collected in very small numbers (as expected in blackwater Coastal Plain streams, Smock et al. 1985) from three MCSC sites. Scrapers were collected from five FBNY sites and no MCSC sites. Among forage fish, common shiner and creek chub were each collected from five of the eight FBNY, sites and shiners were collected from all four of the MCSC sites. Brook trout large enough to be considered predatory game fish (and classified as invertivore/piscivores) were collected from only two FBNY sites; smaller brook trout (classified as invertivores) were collected from four sites. Benthic invertivores (darter species) and predatory game fishes were each collected from three MCSC sites. These data, as well as those of other taxa and (or) feeding groups that were incidentally collected (and included in analysis of biomagnification patterns) are listed in Online Resource #2.
Regional comparison of biotic Hg and trophic position
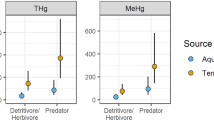
Mean Hg within invertebrate feeding groups that were collected from both study areas were similar between the two regions (Fig. 2a). This was also the case for fish feeding groups, with the notable exception of much lower Hg for invertivorous fish from FBNY than from MCSC (ANOVA F1, 204 = 32, P < 0.0001). When fish were more broadly grouped, mean Hg concentrations were lower for both game fish and forage fish (ANOVA F1, 33 = 7, P 0.015, and F1, 33 = 583, P < 0001, respectively) collected from FBNY (1300 and 560 ng/g, respectively) than MCSC (2300 and 780 ng/g, respectively). The U.S. EPA’s 0.3 μg/g (wet weight basis) MeHg criterion for human health was exceeded in 31% of FBNY game fish specimens and in 80% of MCSC specimens. Similarly, the 0.1 μg/g wildlife guideline was exceeded by a smaller percentage of forage fish specimens collected from FBNY (50%) than MCSC (62%).
a Hg concentrations and b trophic positions of macroinvertebrates and fish from streams in the Fishing Brook, NY (gray boxes) and McTier Creek, SC (white boxes) basins. Each box is based on data pooled among sites (eight NY and four SC) and sampling periods (April–Nov. 2007–2009). Lower and upper boundaries of each box represent 25th and 75th percentiles, lower and upper whiskers represent 10th and 90th percentiles, black dots represent 5th and 95th percentiles, and the line within the box represents the median. Abbreviations are as follows: Hrb-Inv herbivore–invertivore, Inv-Hrb invertivore–herbivore, Invtv invertivore, Inv-Psc invertivore–piscivore, Psc-Inv piscivore–invertivore. Groups that are not significantly different (nsd, alpha = 0.05; ANOVA on log-transformed values followed by Tukey’s HSD test) are indicated as follows: rectangle indicates nsd between study areas for particular organism group; the same letter indicates nsd within regions (letters for NY are above the boxes and letters for SC are below). Numbers of samples are above the x-axis
Shredders exhibited the lowest δ15N among all feeding groups in both study areas (see Online Resource #2) and were the base consumer proxy for trophic position calculations. The resulting median trophic positions of FBNY game fish (3.3) and forage fish (2.7) were 12% lower and 22% slower, respectively, than those of MCSC game fish (3.7), and forage fish (3.3). The large regional difference in game fish and forage fish trophic positions corresponded with observed biotic Hg differences between the regions. Mean trophic positions of all macroinvertebrate and fish feeding groups tested were also significantly lower in FBNY than in MCSC (ANOVA F from 7 to 401, P from 0.013 to <0.0001), even for feeding groups for which Hg did not differ between regions. The trophic position difference was especially large (33% lower, ANOVA F1, 204 = 32, P < 0.0001) in the case of invertivore fish from FBNY (2.7) than MCSC (3.6), and corresponded with the large regional difference in biotic Hg of that feeding group.
The expected pattern of increasing Hg concentration within regions with increasing carnivory of the feeding group was generally similar between the two regions (Fig. 2a), and differences among feeding groups were highly significant (ANOVA F7, 88 = 292, P < 0.0001 for FBNY; F6, 54 = 204, P < 0.0001 for MCSC). Mean Hg concentration of predator invertebrates (secondary consumers) was higher than that of shredders and scrapers (both primary consumers), and mean Hg of forage fish feeding groups were higher than that of predatory invertebrates. Among primary consumers in FBNY, Hg was higher in scrapers than in shredders, and among forage fish groups in FBNY, Hg was higher in the herbivore-invertivore group than in the others. A notable difference between the two study areas is the larger overlap among most fish feeding groups in FBNY (including predatory game fish), than in MCSC.
Hg, δ15N and trophic position spatial patterns
Site-to-site differences in Hg concentrations for macroinvertebrates and for forage fish were relatively large across FBNY (Fig. 3a), and small across MCSC (Fig. 3b). In FBNY, Hg concentrations were generally highest in the Sixmile sites (S2-3), lowest in the high-gradient tributary (UT; shredder macroinvertebrates only), and intermediate in the Fishing Brook sites (F1-3). In addition, biotic Hg was generally lower in the open water section of Fishing Brook (F3), than in F2 (which is well upstream of the open water section, see Fig. 1). Darters and predatory invertebrates from G1, the MCSC tributary site, had significantly lower Hg than those from either mainstem site (Fig. 3b), but there were no discernable patterns for shiners and crayfish.
Site-to-site variation of Hg and nitrogen stable isotope ratio (δ15N) in the Fishing Brook, NY (FBNY) and McTier Creek, SC (MCSC) basins. a and b are Hg in FBNY and MCSC, respectively; c and d are δ15N in FBNY and MCSC, respectively. Lower and upper boundaries of each box represent 25th and 75th percentiles, lower and upper whiskers represent the 10th and 90th percentiles, black dots represent the 5th and 95th percentiles, and the line within the box represents the median. Site names are listed in Table 1. Sites are arranged in order of increasing mean HTD. Groups that are not significantly different (alpha > 0.05; ANOVA on log-transformed values followed by Tukey’s HSD test) are indicated with the same letter. Numbers of samples are shown below each box
Whereas spatial variation of δ15N within feeding groups was generally small across FBNY (Fig. 3a), it was very large across MCSC (Fig. 3b). Shredders collected from the high-gradient, non-wetland FBNY site (site UT) were an exception to this; mean shredder δ15N was nearly an order of magnitude lower than any other FBNY site. The MCSC spatial pattern in δ15N was fairly consistent, with more enriched δ15N at M1, and least at G1, corresponding with the relative amounts of agricultural development in these sub-basins (Table 1).
Hg spatial patterns in relation to stream chemistry and landscape characteristics
The spatial pattern of mean Hg concentrations for most consumer groups collected from FBNY was positively correlated to mean growing-season FMeHg and DOC (Fig. 4). In contrast, there was little discernable pattern in MCSC, largely due to the limited range of conditions across the MCSC sites (Fig. 4). Mean Hg concentrations within FBNY shredder invertebrates, predator invertebrates, and shiner groups were positively correlated to growing-season mean FMeHg concentration, although the shiner regression somewhat exceeded our significance criterion. DOC concentration was positively correlated to Hg in shredders, which were collected across a wider range of DOC than other groups. High DOC (greater than 12 mg/L) was related to high biotic Hg in shiners and predator invertebrates (although their correlations were not significant). There was no significant or consistent relation between pH and Hg in any of the feeding groups in FBNY due to the very limited pH range.
Hg in shredder invertebrates, predator invertebrates, and invertivore–herbivore fishes (shiners, Cyprinidae: Notropis spp. or Luxilus cornutus) in relation to growing season mean FMeHg, DOC, and pH for Fishing Brook, NY (solid triangles) and McTier Creek, SC (open circles) stream sites. Biotic Hg concentrations are arithmetic means of MeHg (invertebrates) or THg (fish) collected during April–November 2007–2009. Chemical concentrations are arithmetic means from base flow samples collected during the same time period. Regression lines and Spearman rank correlations (P) are shown for NY sites
The pattern of consumer Hg across the FBNY study area (Fig. 5) was significantly related to the composition and pattern of the FBNY landscape. Shredder Hg was positively correlated to wetland abundance. There were no significant correlations, however, between wetland abundance and Hg in consumers that were collected across a narrower range of site conditions (i.e., wetland abundance 6–13%). Hg in all three FBNY groups was strongly and inversely correlated to mean HTD (but with an elevated P-value for shiners due to the small number of sites).
Hg in: a shiners, b predatory invertebrates (dragonfly larvae), and c shredder invertebrates (caddisfly larvae) in relation to wetland abundance and mean HTD for NY (triangles) and SC (open circles). Biotic Hg concentrations are arithmetic means of MeHg (invertebrates) or THg (fishes) collected during April–November 2007–2009. Regression lines and Spearman rank correlations (P) are shown for NY sites
Discussion
Inter-basin comparison of biotic Hg
MCSC had much higher Hg in fish, and corresponding increased probability of exceeding human and wildlife advisory levels for forage and game fish (80 and 62%, respectively) compared with FBNY (31 and 50%, respectively). Elevated fish Hg burdens have been widely reported in Coastal Plain streams relative to those in other forested settings across the United States (Scudder et al. 2009) and in other ecoregions within South Carolina (Glover et al. 2010). This pattern corresponded to generally higher trophic position for secondary consumers in MCSC, where the mean predatory game fish trophic position was 3.7 (s.d. 0.32) compared with 3.3 (s.d. 0.34) in FBNY.
Food chain length, or number of trophic positions in the food chain below a predatory fish, is an important factor influencing fish Hg concentrations (Wiener et al. 2003); however, much of the data supporting this comes from studies of lacustrine ecosystems (reviewed in Wiener et al. 2003). Chasar et al. (2009) observed strong correlations between biotic MeHg and δ15N, a surrogate of trophic position, in seven of eight streams across the U.S., although relative trophic position of the fish did not appear to be as important as FMeHg at the base of the food web in explaining biotic Hg patterns across a large environmental gradient (in which mean FMeHg varied by several orders of magnitude). Among three Wisconsin streams, with a more limited range in FMeHg, Chasar et al. (2009) did observe an increase in fish Hg with trophic position. Food web and physico-chemical characteristics can be important drivers of consumer Hg burdens, if differences in food chain length are large relative to differences in MeHg concentrations at the base of the food web (Ward et al. 2010). In this study, the relative difference in food chain length between MCSC and FBNY (i.e., 12% longer in MCSC) was sufficiently large to overcome any potential influence of the lower mean growing season FMeHg in MCSC than FBNY (relative difference 120%), lower DOC (relative difference 48%), lower wetland coverage (relative difference 18%) and the higher HTD (relative difference 17%).
Trophic position and consumer Hg
As expected, trophic position was an important within-site predictor of consumer Hg in both study areas. The increasing biotic Hg with increasing carnivory and piscivory in the diet corresponded with literature-based feeding group classifications in most, but not all, cases. In FBNY, Hg in scrapers was at least twice that in shredders, both for all sites pooled and within sites and sampling dates, although both are considered primary consumers. Tsui et al. (2009) reported higher Hg in scrapers than shredders, related to a higher Hg content of periphyton as compared with detritus. Algal uptake may be an important MeHg entry point for stream food webs, and primary consumption by scrapers, such as Heptageniidae, may be an important pathway of Hg transfer to higher trophic levels (Mason et al. 2000; Castro et al. 2007; Cremona et al. 2009; Ward et al. 2010). In our case, the higher Hg in scrapers may be attributed at least in part to their higher trophic position in relation to shredders, partly a function of the macroinvertebrate family targeted for this feeding group in our study. Higher trophic position estimates for Heptageniidae than other scraper families (and Limnephilidae caddisfly larvae) were also documented by Anderson and Cabana (2007), who suggest this is due to a greater tendency toward omnivory by Heptageniidae than most other scraper families. The high Hg of herbivore-invertivore fish in FBNY compared with other forage fish feeding groups in FBNY also did not agree with what would be expected for this classification, but did correspond with their higher trophic position relative to invertivore–herbivores, which suggests that the northern redbelly dace are consuming a relatively large proportion of invertebrates, and highlights the benefits of incorporating stable isotope data with literature classifications in contaminant studies.
Spatial patterns in biotic Hg
The relative spatial homogeneity of biotic Hg in MCSC corresponds to a general lack of spatial variability in topography or stream geochemistry among study sites. Relatively homogeneous geochemistry for MCSC was also reported by Bradley et al. (2011). Strong relationships between fish Hg burdens, wetland percentage and water chemistry have been reported for South Carolina (Guentzel 2009; Glover et al. 2010), among sites spanning a greater range of ecoregions, waterbody types and sizes, and wetlands coverage (from less than 1% to more than 30%). Local reach and habitat characteristics that were not evaluated in the current study may be important sources of spatial variation in MCSC, potentially explaining the significantly lower biotic Hg in darters and dragonfly larvae from the Gully Creek tributary site. Bradley et al. (2011) report significantly lower aqueous MeHg downstream of a small pond in the MCSC, but biota were not collected from that site for the present study.
Spatial variation in consumer Hg was large across FBNY. This large variation in biotic Hg on a relatively small spatial scale has been reported in Adirondack lakes (Driscoll et al. 1994; Simonin et al. 2008), and across streams within a somewhat larger (150 km2) basin in northern California (Tsui et al. 2009), but has not previously been reported for small Adirondack streams. Bradley et al. (2011) also report large spatial variation in geochemistry across the FBNY study area.
Factors influencing biotic Hg spatial variation in FBNY
The spatial pattern of Hg in FBNY consumers corresponded closely with that of aqueous MeHg and, for some consumer groups, with DOC. A significant relationship between biotic Hg, dissolved MeHg, and DOC has been reported previously over broader geographic scales and across much larger geochemical gradients (e.g., Chasar et al. 2009; Scudder et al. 2009). Some studies report inhibitory effects of DOC on algal uptake and hence, bioaccumulation (Driscoll et al. 1994; Kamman et al. 2005; Gorski et al. 2008); however, DOC concentrations in our study were consistently lower than were those previously reported to inhibit algal uptake of MeHg.
Both DOC and MeHg are produced in riparian wetlands (Driscoll et al. 1994; St. Louis et al. 1994; Krabbenhoft et al. 1995), and DOC export from wetlands to streams facilitates MeHg transport downslope and downstream (Driscoll et al. 1995; Grigal 2002, 2003; Hall et al. 2008). The importance of riparian wetlands as sources of Hg within FBNY was confirmed by Schelker et al. (2011). In the current study, wetland abundance was significantly correlated with MeHg only in shredder macroinvertebrates, the only consumer collected from the full range of wetland abundances, including the site lacking a wetland. Remaining functional groups were collected across a narrower range of wetland coverages—too narrow for wetlands abundance to be a useful predictor of biotic Hg. Our results are similar to those of Ward et al. (2010), who found a 5- to 13-fold range of Hg in stream-dwelling juvenile Atlantic salmon (Salmo salar) across a narrow range of wetland abundance, but no strong relation between biotic Hg and wetland abundance.
The lack of strong and consistent correlations between biotic Hg and wetland abundance in this study (despite a large range in biotic Hg across the FBNY study area) may be due to several factors. Imagery used to produce maps can significantly underestimate wetlands area (Tiner et al. 2002), especially where there is high forest cover (Lindsay et al. 2004). However, in our case we used wetland data from high-quality photogrammetric imagery for the FBNY study area (http://www.apa.state.ny.us/Research/uh/uhreporttitle.html, accessed 7 May 2010). It is more likely that wetland area metrics do not sufficiently quantify small-scale landscape variations that are highly relevant to MeHg production and movement. For example, topographic depressions that are not classified as wetland (i.e., are too small, or too ephemeral for wetland classification based on vegetation) can produce significant amounts of aqueous MeHg and DOC (Grigal 2002; Lindsay et al. 2004). Additionally, the potential production of MeHg and DOC in wetlands and subsequent delivery to streams is strongly influenced by wetland characteristics that are not quantified by wetland abundance, including distance to the stream channel (Grigal 2002), hydrologic connection (St. Louis et al. 1996; Bradley et al. 2010), and wetland type and plant composition (St. Louis et al. 1996).
HTD emerged as a robust predictor of Hg in consumers within the FBNY basin. HTD integrates slope, drainage density, and riparian area. These three basin characteristics affect Hg methylation and transport to the stream. HTD is positively related to slope and negatively related to riparian area and drainage density. Lower mean basin slope and greater riparian area favor greater formation of MeHg, while higher drainage density favors efficient MeHg transport to the stream in low-slope areas. Most of the transport of MeHg to streams in the FBNY basins is thought to be via shallow groundwater flow from flat-lying riparian areas where wetlands are common (Schelker et al. 2011). Thus, decreasing HTD across the sub-basins in FBNY reflects both an increase in factors associated with MeHg and DOC sources as well as a decrease in the transport distance of these constituents to the stream habitat. Because the life histories and behaviors of some organisms are timed to take advantage of the habitat and high-quality food sources available in seasonally flooded riparian wetlands (Resh et al. 1988), lower HTD may also indicate greater opportunity for mobile macroinvertebrates, such as Limnephillidae caddisflies, to feed in wetland habitats.
The superior predictive ability of a topographic-based metric over simple wetland abundance has been noted elsewhere for aqueous MeHg and for DOC, but not for Hg in stream biota. Dennis et al. (2005) used terrain analysis of digital elevation data to determine an “soil drainage index” for predicting base-flow MeHg in Adirondack lakes. Lindsay et al. (2004) found “bottomland wetland area” (a Digital Elevation Map—derived metric that integrates extent, position, and connectivity of wetlands to a stream) to be an important control on runoff dynamics for forested catchments on the Canadian Shield. Canham et al. (2004) modeled DOC concentrations in Adirondack lakes with flow-path distances for particular land covers; and Frost et al. (2006) found that drainage density, slope, and stream length each were negatively related to DOC concentration and, together, accounted for 44% of the variation in DOC across a northwestern Wisconsin watershed.
Other local reach characteristics may contribute to spatial variability in Hg in surface waters and biota. We found consistently lower Hg in consumers collected from the shallow, open water portion of FBNY (F3, County Line Flow) than from the site immediately upstream. This pattern corresponds with documented lower concentrations of aqueous MeHg and DOC in the County Line Flow (Bradley et al. 2011; Schelker et al. 2011). Aqueous MeHg loss in this reach may be due to sedimentation of particle-bound MeHg when going from channelized flow to shallow open water, and/or to demethylation by photoreduction (Bradley et al. 2011). The open water bodies in the FBNY basin include natural lakes such as Pickwacket Pond (upstream of P2) and a shallow ‘run-of-the-river’ impoundment, County Line Flow (F3), which has a high flushing rate and was constructed nearly a century ago. Other types of impoundments, such as younger reservoirs (e.g., Hecky et al. 1991) and beaver impoundments, which are common throughout the Adirondacks and Coastal Plain, may affect Hg cycling and bioaccumulation differently at the reach scale (Driscoll et al. 1995, 1998; Selvendiran et al. 2008; Roy et al. 2009).
Conclusions
The spatial patterns of Hg in consumers in the two contrasting basins in this study can be attributed to food web and physico-chemical factors. The relative importance of each factor differs, however, depending on the geographic scale of interest. At the regional scale, comparing Hg bioaccumulation in consumers of two widely separated areas with very different landscapes, we found that food web processes, particularly food chain length, are the main predictors of the regional differences in consumer Hg. On a local scale, we found that physico-chemical and landscape processes were the main predictors of biotic Hg in the topographically heterogeneous setting of the Adirondacks. The large within-region variability in Hg among different macroinvertebrate feeding groups and among different forage fish taxa emphasized the importance of considering detailed feeding ecologies, and the benefits of incorporating stable isotope data, for spatial comparisons at local scales.
Landscape metrics can predict Hg bioaccumulation and its spatial variation across small watersheds, as they do across larger geographic extents. Our results demonstrate that local-scale patterns of Hg concentrations in stream biota are strongly associated with hydro-geomorphology and landscape characteristics that influence MeHg production and delivery to streams. Important characteristics are short flow paths from topographic depressions to the stream channel, closely associated riparian wetlands, and elevated concentrations of aqueous MeHg and DOC. Within the topographically heterogeneous Adirondack region of NY, biotic Hg concentrations vary significantly over a relatively small spatial scale. In contrast, within the more homogeneous, flat Coastal Plain of SC, with less variable terrain and water chemistry, Hg concentrations in aquatic biota did not demonstrate any apparent relation with landscape characteristics at a similar spatial scale. Our results highlight the importance of considering local-scale spatial variability when assessing sensitivity of lotic ecosystems to Hg deposition in the geographically extensive Adirondack region of NY and the Coastal Plain region of the southeastern US. These findings have implications for the development of monitoring plans for Hg in water or biota, the ranking of Hg-sensitive areas, and the use of readily available landscape data. Hydro-geomorphic metrics such as HTD can be robust predictors of Hg in streams and stream biota within the heterogeneous Adirondack landscape and similar regions, and may be a more sensitive indicator than wetland density in topographically heterogeneous settings across limited ranges of wetland cover.
References
Anderson C, Cabana G (2007) Estimating the trophic position of aquatic consumers in riverine food webs using nitrogen stable isotopes. J N Am Benthol Soc 26:273–285
Beasley BR, Marshall WD, Miglarese AH, Scurry JD, Vanden Houten CE (1996) Managing resources for a sustainable future: the Edisto River Basin project report, No. 12. SC Department of Natural Resources, Water Resources Division, Columbia
Bloom NS (1992) On the chemical form of mercury in edible fish and marine invertebrate tissue. Can J Fish Aquat Sci 49:1010–1017
Bradley PM, Journey CA, Chapelle FH, Lowery MA, Conrads PA (2010) Flood hydrology and methylmercury availability in Coastal Plain rivers. Environ Sci Technol 44:9285–9290
Bradley PM, Burns DA, Riva-Murray K, Brigham ME, Button DT, Chasar LC, Marvin-DiPasquale M, Lowery MA, Journey CA (2011) Spatial and seasonal variability of dissolved methylmercury in two stream basins in the eastern United States. Environ Sci Technol 45:2048–2055
Brigham ME, Wentz DA, Aiken GR, Krabbenhoft DP (2009) Mercury cycling in stream ecosystems. 1. Water column chemistry and transport. Environ Sci Technol 43:2720–2725
Brumbaugh WG, Krabbenhoft DP, Helsel DR, Wiener JG, Echols KR (2001) A national pilot study of mercury contamination of aquatic ecosystems along multiple gradients: bioaccumulation in fish, Biol Sci Rep USGS/BRD/BSR-2001-0009, U.S. Geological Survey, Reston
Canham CD, Pace ML, Papaik MJ, Primack AGB, Roy KM, Maranger RJ, Curran RP, Spada DM (2004) A spatially explicit watershed-scale analysis of dissolved organic carbon in Adirondack lakes. Ecol Appl 14:839–854
Castro MS, Hilderbrand RH, Thompson H, Heft A, Rivers SE (2007) Relationship between wetlands and mercury in brook trout. Arch Environ Contam Toxicol 52:97–103
Chasar LC, Scudder BC, Stewart AR, Bell AH, Aiken GR (2009) Mercury cycling in stream ecosystems. 3. Trophic dynamics and methylmercury bioaccumulation. Environ Sci Technol 43:2733–2739
Chen CY, Folt CL (2005) High plankton densities reduce mercury biomagnifications. Environ Sci Technol 39:115–121
Cimmery V (ed) (2007) User guide for SAGA Version 2.0. http://sourceforge.net/projects/saga-gis/files/. Accessed 8 March 2010
Cremona F, Hamelin S, Planas D, Lucotte M (2009) Sources of organic matter and methylmercury in littoral macroinvertebrates: a stable isotope approach. Biogeochemistry 94:81–94
Dennis IF, Clair TA, Driscoll CT, Kamman N, Chalmers A, Shanley J, Norton SA, Kahl S (2005) Distribution patterns of mercury in lakes and rivers of northeastern North America. Ecotoxicology 14:113–123
Driscoll CT, Yan C, Schofield CL, Munson R, Holsapple J (1994) The mercury cycle and fish in the Adirondack lakes. Environ Sci Technol 28:136–143
Driscoll CT, Blette V, Yan C, Schofield CL, Munson R, Holsapple J (1995) The role of dissolved organic carbon in the chemistry and bioavailability of mercury in remote Adirondack lakes. Water Air Soil Pollut 80:499–508
Driscoll CT, Holsapple J, Schofield CL, Munson R (1998) The chemistry and transport of mercury in a small wetland in the Adirondack Region of New York, USA. Biogeochemistry 40:137–146
Driscoll CT, Han Y, Chen CY, Evers DC, Lambert KF, Holsen TM, Kamman NC, Munson RK (2007) Mercury contamination in forest and freshwater ecosystems in the northeastern United States. Bioscience 57:17–43
Evers DC, Han Y, Driscoll CT, Kamman NC, Goodale MW, Lambert KF, Holsen TM, Chen CY, Clair TA, Butler T (2007) Biological mercury hotspots in the northeastern United States and Southeastern Canada. Bioscience 57:29–43
Fitzgerald WF, Engstrom DR, Mason RB, Nater EA (1998) The case for atmospheric mercury contamination in remote areas. Environ Sci Technol 32:1–7
Frost PC, Larson JH, Johnston CA, Young KC, Maurice PA, Lamberti GA, Bridgham SD (2006) Landscape predictors of stream dissolved organic matter concentrations and physicochemistry in a Lake Superior river watershed. Aquat Sci 68:40–51
Glover JB, Domino ME, Altman KC, Dillman JW, Castleberry WS, Eidson JP, Mattocks M (2010) Mercury in South Carolina fishes, USA. Ecotoxicology 19:781–795
Gorski PR, Armstrong DE, Hurley JP, Krabbenhoft DP (2008) Influence of natural dissolved organic carbon on the bioavailability of mercury to a freshwater alga. Environ Pollut 154:116–123
Grieb TM, Driscoll CT, Gloss SP, Schofield CL, Bowie GL, Porcella DB (1990) Factors affecting mercury accumulation in fish in the upper Michigan peninsula. Environ Toxicol Chem 9:919–930
Grigal DF (2002) Inputs and outputs of mercury from terrestrial watersheds: a review. Environ Rev 10:1–39
Grigal DF (2003) Mercury sequestration in forests and peatlands: a review. J Environ Qual 32:393–405
Guentzel JL (2009) Wetland influences on mercury transport and bioaccumulation in South Carolina. Sci Total Environ 407:1344–1353
Hall BD, Aiken GR, Krabbenhoft DP, Marvin-DiPasquale M, Swarzenski CM (2008) Wetlands as principal zones of methylmercury production in southern Louisiana and the Gulf of Mexico region. Environ Pollut 154:124–134
Hammerschmidt CR, Fitzgerald WF (2005) Methylmercury in mosquitoes related to atmospheric mercury deposition and contamination. Environ Sci Technol 39:3034–3039
Hecky RE, Ramsey DJ, Bodaly RA, Strange NE (1991) Increased methylmercury contamination in fish in newly formed freshwater reservoirs. In: Suzuki T, Nibumasa I, Clarkson T (eds) Advances in mercury toxicology. Plenum Press, New York, pp 33–52
Helsel DR (2005) Nondetects and data analysis. Wiley, New York
Kamman NC, Burgess NM, Driscoll CT, Simonin HA, Goodale W, Linehan J, Estabrook R, Hutcheson M, Major A, Scheuhammer AM, Scruton DA (2005) Mercury in freshwater fish of northeast North America—a geographic perspective based on fish tissue monitoring databases. Ecotoxicology 14:163–180
Kolka RK, Nater EA, Grigal DF, Verry ES (1999) Atmospheric inputs of mercury and organic carbon into a forested upland/bog watershed. Water Air Soil Pollut 113:273–294
Krabbenhoft DP, Benoit JM, Babiarz CL, Hurley JP, Andren AW (1995) Mercury cycling in the Allequash Creek Watershed, northern Wisconsin. Water Air Soil Pollut 80:425–433
Lindberg S, Bullock R, Ebinghaus R, Engstrom D, Feng X, Fitzgerald W, Pirrone N, Prestbo E, Seigneur C (2007) A synthesis of progress and uncertainties in attributing the sources of mercury in deposition. Ambio 36:19–32
Lindsay JB, Creed IF, Beall FD (2004) Drainage basin morphometrics for depressional landscapes. Water Resour Res 30:W09307. doi:10.1029/2004WR003322
Mason RP, Reinfelder JR, Morel FMM (1995) Bioaccumulation of mercury and methylmercury. Water Air Soil Pollut 80:915–921
Mason RP, Laporte JM, Andres S (2000) Factors controlling the bioaccumulation of mercury, methylmercury, arsenic, selenium, and cadmium by freshwater invertebrates and fish. Arch Environ Contam Toxicol 38:283–297
Merritt RW, Cummins KW (1996) An introduction to the aquatic insects of North America, 3rd edn. Kendall/Hunt Publishing Co., Dubuque
NYDOH (2010) Chemicals in sportfish and game 2010–2011 health advisories. New York State Department of Health. http://www.nyhealth.gov/environmental/outdoors/fish/docs/. Accessed 13 Sep 2010
Post DM (2002) The long and short of food-chain length. Trends Ecol Evol 17:269–277
Post DM, Layman CA, Arrington DA, Takimoto G, Quattrochi J, Montana CG (2007) Getting to the fat of the matter: models, methods, and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152:179–189
Resh VH, Brown AV, Covich AP, Gurtz ME, Li HW, Minshall GW, Reice SR, Sheldon AL, Wallace JB, Wissmar R (1988) The role of disturbance in stream ecology. J N Am Benthol Soc 7:433–455
Rohde FC, Arndt RG, Lindquist DG, Parnell JF (1994) Freshwater fishes of the Carolinas, Virginia, Maryland, and Delaware. University of North Carolina Press, Chapel Hill
Roy V, Amyot M, Carignan R (2009) Beaver ponds increase methylmercury concentrations in Canadian shield streams along vegetation and pond-age gradients. Environ Sci Technol 43:5605–5611
Rypel AL, Arrington DA, Findlay RH (2008) Mercury in southeastern U.S. riverine fish populations linked to water body type. Environ Sci Technol 42:5118–5124
SCDHEC (2010) South Carolina fish consumption advisories. http://www.scdhec.gov/environment/water/fish. Accessed 13 Sep 2010
Schelker J, Burns DA, Weiler M, Laudon H (2011) Hydrological mobilization of mercury and dissolved organic carbon in a snow-dominated forested watershed–conceptualization and modeling. J Geophys Res 116:G01002. doi:10.1029/2010JG001330
Scudder BC, Chasar LC, DeWeese LR, Brigham ME, Wentz DA, Brumbaugh WG (2008) Procedures for collecting and processing aquatic invertebrates and fish for analysis of mercury as part of the National Water-Quality Assessment Program: U.S. Geological Survey Open-File Report 2008-1208
Scudder BC, Chasar LC, Wentz DA, Bauch NJ, Brigham ME, Moran PW, Krabbenhoft DP (2009) Mercury in fish, bed sediment, and water from streams across the United States, 1998–2005. U.S. Geological Survey Scientific Investigations Report 2009–5109
Selvendiran P, Driscoll CT, Bushey JT, Montesdeoca MR (2008) Wetland influence on mercury fate and transport in a temperate forested watershed. Environ Pollut 154:46–55
Simonin HA, Loukmas JJ, Skinner LC, Roy KM (2008) Lake variability: key factors controlling mercury concentrations in New York State fish. Environ Pollut 154:107–115
Smith CL (1985) The inland fishes of New York. New York Department of Environmental Conservation, Albany
Smock LA, Gilinsky E, Stoneburner DL (1985) Macroinvertebrate production in a Southeastern U.S.A. blackwater stream. Ecology 66:1491–1503
St. Louis VL, Rudd JWM, Kelly CA, Beatty KG, Bloom NS, Flett RJ (1994) Importance of wetlands as sources of methylmercury to boreal forest ecosystems. Can J Fish Aquat Sci 51:1065–1076
St. Louis VL, Rudd JWM, Kelly CA, Beatty KG, Flett RJ, Roulet NT (1996) Production and loss of methylmercury and loss of total mercury from boreal forest catchments containing different types of wetlands. Environ Sci Technol 30:2719–2729
Thorp JH, Covich AP (eds) (2001) Ecology and classification of North American freshwater invertebrates, 3rd edn. Academic Press, New York
Tiner RW, Bergquist HC, DeAlessio GP, Starr MJ (2002) Geographically isolated wetlands: a preliminary assessment of their characteristics and status in selected regions of the United States. U.S. Fish and Wildlife Service, Northeast Region, Hadley
Tsui MTK, Finlay JC, Nater EA (2009) Mercury bioaccumulation in a stream network. Environ Sci Technol 43:7016–7022
U.S. Environmental Protection Agency (USEPA) (2009) The national study of chemical residues in lake fish tissue. EPA-823-R-09-006. U.S. Environmental Protection Agency, Office of Water, Washington. http://www.epa.gov/waterscience/fish/study/data/finalreport.pdf. Accessed 3 Nov 2010
Ward DM, Nislow KH, Folt CL (2010) Bioaccumulation syndrome: identifying factors that make some stream food webs prone to elevated mercury bioaccumulation. Ann NY Acad Sci 1195:62–83
Wiener JG, Krabbenhoft DP, Heinz GH, Scheuhammer AM (2003) Ecotoxicology of mercury. In: Hoffman DJ, Rattner BA, Burton GA Jr, Cairns J Jr (eds) Handbook of ecotoxicology, 2nd edn. CRC Press, Boca Raton, pp 409–463
Wiener JG, Knights BC, Sandheinrich MB, Jeremiason JD, Brigham ME, Engstrom DR, Woodruff LG, Cannon WF, Balogh SJ (2006) Mercury in soils, lakes, and fish in Voyageurs National Park (Minnesota): importance of atmospheric deposition and ecosystem factors. Environ Sci Technol 40:6261–6268
Acknowledgments
The authors are grateful to many who collected and processed samples in field and lab, led by John Byrnes, Celeste Journey, Whitney Springfield, John DeWild (all USGS), and Bob Taylor (Texas A&M University). We thank Dan Button and Tia Stevens (both USGS) for data management; Doug Carlsen, AJ Smith, and Brian Duffy (NYSDEC), and Rick Morse and Bryan Weatherwax (New York State Museum) for technical advice and assistance; and the staff of the Adirondack Ecological Center in Newcomb, NY for use of their field laboratory. We are grateful for access provided by landowners—Finch-Pruyn, The Nature Conservancy, and RMK Timberland (Fishing Brook), and the Strom Thurmond family (McTier Creek). We appreciate the very helpful comments of three anonymous reviewers, and reviews of an earlier draft by Barb Scudder Eikenberry and Chris Schmitt. This research was supported by the U.S. Geological Survey National Water Quality Assessment Program. The use of trade, product, or firm names in this article is for descriptive purposes only and does not imply endorsement by the U.S. government.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Thomas A. Abrahamsen—retired.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Riva-Murray, K., Chasar, L.C., Bradley, P.M. et al. Spatial patterns of mercury in macroinvertebrates and fishes from streams of two contrasting forested landscapes in the eastern United States. Ecotoxicology 20, 1530–1542 (2011). https://doi.org/10.1007/s10646-011-0719-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-011-0719-9