Abstract
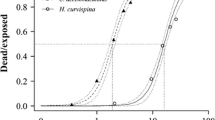
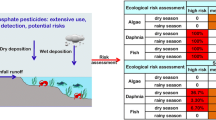
Endosulfan is an insecticide–acaricide used in South Florida and is one of the remaining organochlorine insecticides registered under the Federal Insecticide Fungicide and Rodenticide Act by the U.S.EPA. The technical grade material consists of two isomers (α-, β-) and the main environmental metabolite in water, sediment and tissue is endosulfan sulfate through oxidation. A comprehensive probabilistic aquatic ecological risk assessment was conducted to determine the potential risks of existing exposures to endosulfan and endosulfan sulfate in freshwaters of South Florida based on historical data (1992–2007). The assessment included hazard assessment (Tier 1) followed by probabilistic risk assessment (Tier 2). Tier 1 compared actual measured concentrations in surface freshwaters of 47 sites in South Florida from historical data to U.S.EPA numerical water quality criteria. Based on results of Tier 1, Tier 2 focused on the acute and chronic risks of endosulfan at nine sites by comparing distributions of surface water exposure concentrations of endosulfan [i.e., for total endosulfan (summation of concentrations of α- and β-isomers plus the sulfate), α- plus β-endosulfan, and endosulfan sulfate (alone)] with distributions of species effects from laboratory toxicity data. In Tier 2 the distribution of total endosulfan in fish tissue (whole body) from South Florida freshwaters was also used to determine the probability of exceeding a distribution of whole body residues of endosulfan producing mortality (critical lethal residues). Tier 1 showed the majority of endosulfan water quality violations in South Florida were at locations S-178 followed by S-177 in the C-111 system (southeastern boundary of Everglades National Park (ENP)). Nine surface water sampling sites were chosen for Tier 2. Tier 2 showed the highest potentially affected fraction of toxicity values (>10%) by the estimated 90th centile exposure concentration (total endosulfan) was at S-178. At all other freshwater sites there were <5% of the toxicity values exceeded. Potential chronic risk (9.2% for total endosulfan) was only found at S-178 and all other sites were <5%. Joint probability curves showed the higher probability of risk at S-178 than at S-177. The freshwater fish species which contain tissue concentrations of endosulfan (total) with the highest potential risk for lethal whole body tissue residues were marsh killifish, flagfish and mosquitofish. Based on existing surface water exposures and available aquatic toxicity data, there are potential risks of total endosulfan to freshwater organisms in South Florida. Although there are uncertainties, the presence of tissue concentrations of endosulfan in small demersal fish, is of ecological significance since these fish support higher trophic level species, such as wading birds.





Similar content being viewed by others
References
Agency for Toxic Substances, Disease Registry (ATSDR) (2000) Toxicological profile for endosulfan. US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Atlanta
Aldenberg T, Jaworska JS, Traas TP (2002) Normal species sensitivity distributions and probabilistic ecological risk assessment. In: Posthuma L, Suter GW, Traas TP (eds) Species sensitivity distributions in ecotoxicology. Lewis Publishers, Boca Raton, FL, pp 49–102
Berntssen MHG, Glover CN, Robb DHF, Jakobsen JV, Petri D (2008) Accumulation and elimination kinetics of dietary endosulfan in Atlantic salmon (Salmo salar). Aquat Toxicol 86:104–111
Carriger JF, Rand GM (2008a) Aquatic risk assessment of pesticides in surface waters in and adjacent to the Everglades and Biscayne National Parks: I. Hazard assessment and problem formulation. Ecotoxicol 17:660–679
Carriger JF, Rand GM (2008b) Aquatic risk assessment of pesticides in surface waters in and adjacent to the Everglades and Biscayne National Parks: II. Probabilistic analyses. Ecotoxicol 17:680–696
Carriger JF, Rand GM, Gardinali PR, Perry WB, Tompkins MS, Fernandez AM (2006) Pesticides of potential ecological concern in sediment from South Florida canals: an ecological risk prioritization for aquatic arthropods. Soil Sediment Contam 15:21–45
Chapman PM, Caldwell RS, Chapman PF (1996) A warning: NOECs are inappropriate for regulatory use. Environ Toxicol Chem 15:77–79
Cotham WE, Bidleman TF (1989) Degradation of malathion, endosulfan, and fenvalerate in seawater and seawater sediment microcosms. J Agric Food Chem 37:824–828
Crane M, Newman MC (2000) What level of effect is a no observed effect? Environ Toxicol Chem 19:516–519
De Zwart D (2002) Observed regularities in species sensitivity distributions for aquatic species. In: Posthuma L, Suter GW, Traas TP (eds) Species sensitivity distributions in ecotoxicology. Lewis Publishers, Boca Raton, pp 133–154
ECOFRAM (1999) Ecological committee on FIFRA risk assessment methods: report of the aquatic workgroup. U.S. Environmental Protection Agency, Office of Pesticide Programs, Washington, DC
Escher BI, Hermens JLM (2002) Modes of action in ecotoxicology: their role in body burdens, species sensitivity, QSARs, and mixture effects. Environ Sci Technol 36:4201–4217
Escher BI, Hermens JLM (2004) Internal exposure: linking bioavailability to effects. Environ Sci Technol 38:6263–6270
Ffrench-Constant RH (1993) The molecular and population genetics of cyclodiene insecticide resistence. Insect Biochem Mol Biol 24:335–345
Ffrench-Constant RH, Anthony N, Aronstein K, Rocheleau T, Stilwell G (2000) Cyclodiene insecticide resistance: from molecular to population genetics. Annu Rev Entomol 48:449–466
Florida Department of Agriculture, Consumer Services (FDACS) (2003) Summary of agricultural pesticide usage in Florida: 1999–2002. FDACS, Division of Agricultural Environmental Services, Bureau of Pesticides, Tallahassee
Fulton MH, Scott GI, DeLorenzo ME, Key PB, Bearden DW, Strozier ED, Madden CJ (2004) Surface water pesticide movement from the Dade County agricultural area to the Everglades and Florida Bay via the C-111 canal. Bull Environ Contam Toxicol 73:527–534
GAO (2007) Some restoration progress has been made, but the effort faces significant delays, implementation challenges, and rising costs. United States Government Accountability Office, GAO-07-1250T, Testimony Before the Subcommittee on International Operations and Organizations, Democracy and Human Rights, Committee on Foreign Relations, U.S. Senate, Washington DC
German Federal Environment Agency (GFEA) (2007) Endosulfan: draft dossier prepared in support of a proposal of endosulfan to be considered as a candidate for inclusion in the Annexes to the Stockholm Convention. Umweltbundesamt
Giddings JM, Hall LW, Solomon KR (2000) Ecological risks of diazinon from agricultural use in the Sacramento-San Joaquin River Basins, California. Risk Anal 20:545–572
Giddings JM, Hall LW, Anderson TA, Hosmer AJ, Kendall RJ (2005) Atrazine in North American surface waters: a probabilistic aquatic ecological risk assessment. Society of Environmental Toxicology and Chemistry, Pensacola
Goebel H, Gorbach S, Knauf W, Rimpau RH, Huttenbach H (1982) Properties, effects, residues, and analytics of the insecticide endosulfan. Residue Rev 83:1–165
Goodman LR, Lewis MA, Macauley JM, Smith R Jr, Moore JC (1999) Preliminary survey of chemical contaminants in water, sediment, and aquatic biota at selected sites in Northeastern Florida Bay and canal C-111. Gulf Mex Sci 17:1–16
Gormley KL, Teather KL (2003) Developmental, behavioral, and reproductive effects experienced by Japanese medaka (Oryzias latipes) in response to short-term exposure to endosulfan. Ecotoxicol Environ Saf 54:330–338
Guerin TF (2001) Abiological loss of endosulfan and related chlorinated organic compounds from aqueous systems in the presence and absence of oxygen. Environ Pollut 115:219–230
Hall LW, Gardinali P (2004) Ecological risk assessment for Irgarol 1051 and its major metabolite in United States surface waters. Hum Ecol Risk Assess 10:525–542
Hall LW, Scott MC, Killen WD (1998) Ecological risk assessment of copper and cadmium in surface waters of Chesapeake Bay watershed. Environ Toxicol Chem 17:1172–1189
Hall LW, Anderson RD, Kilian J, Tierney DP (1999) Concurrent exposure assessments of atrazine and metolachlor in the mainstem, major tributaries and small streams of the Chesapeake Bay watershed: indicators of ecological risk. Environ Monit Assess 59:155–190
Hanson ML, Solomon KR (2002) New technique for estimating thresholds of toxicity in ecological risk assessment. Environ Sci Technol 36:3257–3264
Harman-Fetcho JA, Hapeman CJ, McConnell LL, Potter TL, Rice CP, Sadeghi AM, Smith RD, Bialek K, Sefton KA, Schaffer BA, Curry R (2005) Pesticide occurrence in selected South Florida canals and Biscayne Bay during high agricultural activity. J Agric Food Chem 53:6040–6048
Hassall KA (1990) The biochemistry and uses of pesticides structure, metabolism, mode of action and uses in crop protection, 2nd edn. Macmillan Press Ltd, London
Holdway DA, Hefferman J, Smith A (2008) Multigeneration assessment of nonylphenol and endosulfan using a model Australian freshwater fish, Melanotaenia fluviatilis. Environ Toxicol 23:253–262
Hose GC, Van den Brink PJ (2004) Confirming the species-sensitivity distribution concept for endosulfan using laboratory, mesocosm, and field data. Arch Environ Contam Toxicol 47:511–520
IRAC (Insecticide Resistance Action Committee) (2007) IRAC mode of action classification version 5.3. www.irac-online.org
Jarvinen AW, Ankley GT (1999) Linkage of effects to tissue residues: development of a comprehensive database for aquatic organisms exposed to inorganic and organic chemicals. SETAC Technical Publication Series, SETAC Press. Society of Environmental Toxicology and Chemistry, Pensacola
Klepper O, van de Meent D (1997) Mapping the potentially affected fraction (PAF) of species as an indicator of generic toxic stress. RIVM report 607504 001. National Institute of Public Health and the Environment, Bilthoven
Laabs V, Wehrhan A, Pinto A, Dores E, Amelung W (2007) Pesticide fate in tropical wetlands of Brazil: an aquatic microcosm study under semi-field conditions. Chemosphere 67:975–989
Latre B, Ramos MJ (2009) Draft initial detailed risk profile for endosulfan. Prepared for the European Commission, DG Environment, Stockholm Convention Secretariate. http://chm.pops.int/Convention/POPsReviewCommittee/Meetings/POPRC4/AnnexEinformationrequest/tabid/437/language/en-US/Default.aspx. Accessed 17 April 2009
Ley JA, Montague CL, McIvor CC (1994) Food habits of mangrove fishes: a comparison along estuarine gradients in northeastern Florida Bay. Bull Mar Sci 54:881–889
Lorenz JJ, Serafy JE (2006) Subtropical wetland fish assemblages and changing salinity regimes: implications for Everglades restoration. Hydrobiologia 569:401–422
Matthiessen P, Fox PJ, Douthwaite RJ, Wood AB (1982) Accumulation of endosulfan residues in fish and their predators after aerial spraying for the control of tsetse-fly in Botswana. Pestic Sci 13:39–48
McCarty LS, Mackay D (1993) Enhancing ecotoxicological modeling and assessment: body residues and modes of toxic action. Environ Sci Technol 27:1719–1728
Meador JP (2006) Rationale and procedures for using the tissue-residue approach for toxicity assessment and determination of tissue, water, and sediment quality guidelines for aquatic organisms. Hum Ecol Risk Assess 12:1018–1073
Miles JRW, Moy P (1979) Degradation of endosulfan and its metabolites by a mixed culture of soil-microorganisms. Bull Environ Contam Toxicol 23:13–19
Miles CJ, Pfeuffer RJ (1997) Pesticides in canals of South Florida. Arch Environ Contam Toxicol 32:337–345
Moore DRJ, Caux P-Y (1997) Estimating low toxic effects. Environ Toxicol Chem 16:794–801
National Research Council of Canada (NRCC) (1975) Endosulfan: its effects on environmental quality. NRCC publication no. 14098 (1975), NRC Associate Committee on Scientific Criteria for Environmental Quality, Subcommittee on Pesticides and Related Compounds, Ottawa
Navarro S, Barba A, Segura JC, Oliva J (2000) Disappearance of endosulfan residues from seawater and sediment under laboratory conditions. Pestic Manag Sci 56:849–854
Newman MC (1995) Quantitative methods in aquatic ecotoxicology. Lewis Publishers, Boca Raton
Newman MC, Crane M, Holloway G (2006) Does pesticide risk assessment in the European Union assess long-term effects? Rev Environ Contam Toxicol 187:1–65
Pablo F, Hyne RV (2009) Endosulfan application to a stream mesocosm: studies on fate, uptake into passive samplers and caged toxicity test with the fish M. ambigua. Arch Environ Contam Toxicol 56:525–535
Pait AS, DeSouza AE, Farrow DRG (1992) Agricultural pesticide use in coastal areas: a national summary. U.S. Department of Commerce, National Oceanic and Atmospheric Administration, Rockville
Parkpian P, Anurakpongsatorn P, Pakkong P, Patrick WH (1998) Adsorption, desorption and degradation of alpha-endosulfan in tropical soils of Thailand. J Environ Sci Health B-Pestic Food Contam Agric Wastes 33:211–233
Schimmel SC, Patrick Jr JM, Wilson Jr AJ (1977) Acute toxicity to and bioconcentration of endosulfan by estuarine animals. In: Mayer FL, Hamelink JL (eds) Aquatic toxicology and hazard evaluation. American Society for Testing and Materials, USA, pp 241–252, ASTM STP 634
Peterson SM, Batley GE (1993) The fate of endosulfan in aquatic ecosystems. Environ Pollut 82:143–152
Pfeuffer RJ, Rand GM (2004) South Florida ambient pesticide monitoring program. Ecotoxicol 13:195–205
Rand GM, Gardinali PR (2004) South Florida ecosystems. Ecotoxicol 13:179–184
Schuler LJ, Rand GM (2008) Aquatic risk assessment of herbicides in freshwater ecosystems of South Florida. Arch Environ Contam Toxicol 54:571–583
Science Subgroup (1996) South Florida ecosystem restoration: scientific information needs. A Science Subgroup report to the Working Group of the South Florida Ecosystem Restoration Task Force, Miami
Scott GI, Fulton MH, Daugomah J, Strozier ED, Key PB, Pennington PL, Thompson BC, Wirth EF, Thayer G (1994) Monitoring of pesticides in surface waters of Florida Bay and adjacent agricultural watersheds: implications for future management of freshwater inputs to Florida Bay. U.S. National Marine Fisheries Service, Charleston
Scott GI, Fulton MH, Wirth EF, Chandler GT, Key PB, Daugomah JW, Bearden D, Chung KW, Strozier ED, DeLorenzo M, Sivertsen S, Dias A, Sanders M, Macauley JM, Goodman LR, LaCroix MW, Thayer GW, Kucklick J (2002) Toxicological studies in tropical ecosystems: an ecotoxicological risk assessment of pesticide runoff in South Florida estuarine ecosystems. J Agric Food Chem 50:4400–4408
SETAC (Society of Environmental Toxicology and Chemistry) (1994) Pesticide risk and mitigation. Final report of the Aquatic Risk Assessment and Mitigation Dialog Group. SETAC Foundation for Environmental Education, Pensacola
Shen L, Wania F, Lei YD, Teixeira C, Muir DCG, Bidleman TF (2005) Atmospheric distribution and long-range transport behavior of organochlorine pesticides in North America. Environ Sci Technol 39:409–420
Shivaramaiah HM, Sanchez-Bayo F, Al-Rifai J, Kennedy IR (2005) The fate of endosulfan in water. J Environ Sci Health B-Pestic Food Contam Agric Wastes 40:711–720
Solomon KR, Baker DB, Richards RP, Dixon DR, Klaine SJ, LaPoint TW, Kendall RJ, Weisskopf CP, Giddings JM, Giesy JP, Hall LW, Williams WM (1996) Ecological risk assessment of atrazine in North American surface waters. Environ Toxicol Chem 15:31–74
Solomon K, Giesy J, Jones P (2000) Probabilistic risk assessment of agrochemicals in the environment. Crop Prot 19:649–655
Solomon KR, Giddings JM, Maund SJ (2001) Probabilistic risk assessment of cotton pyrethroids: I. Distributional analyses of laboratory aquatic toxicity data. Environ Toxicol Chem 20:652–659
Sparling DW, Fellers GM (2009) Toxicity of two insecticides to California, USA, Anurans and its relevance to declining amphibian populations. Environ Toxicol Chem 28:1696–1703
Sutherland TD, Horne I, Lacey MJ, Harcourt RL, Russell RJ, Oakeshott JG (2000) Enrichment of an endosulfan-degrading mixed bacterial culture. Appl Environ Microbiol 66:2822–2828
Sutherland TD, Horne I, Weir KM, Russell RJ, Oakeshott JG (2004) Toxicity and residues of endosulfan isomers. Rev Environ Contam Toxicol 183:99–113
Systat (2008) SigmaPlot 11.0. Build 11.1.0.102. Systat Software, Inc., Chicago
Traas TP, van de Meent D, Posthuma L, Hamers T, Kater BJ, de Zwart D, Aldenberg T (2002) The potentially affected fraction as a measure of ecological risk. In: Posthuma L, Suter GW, Traas TP (eds) Species sensitivity distributions in ecotoxicology. Lewis Publishers, Boca Raton, FL, pp 315–344
U.S.EPA (1980) Ambient water quality criteria for endosulfan. EPA 440/5-80-046. United States Environmental Protection Agency, Office of Water, Washington, DC
U.S.EPA (1998) Guidelines for ecological risk assessment. EPA/630/R-95/002F. United States Environmental Protection Agency, Office of Water, Washington, DC
U.S.EPA (2002) Environmental fate and ecological risk assessment for the reregistration eligibility decision on endosulfan (Thiodan ®). DP Barcode D238673. United States Environmental Protection Agency, Office of Pesticide Programs, Environmental Fate and Effects Division, Washington, DC
U.S.EPA (2007) Appendix 1 to 2007 addendum: environmental fate and ecological risk assessment of endosulfan. United States Environmental Protection Agency, Office of Pesticide Programs, Washington, DC
Van de Plassche EJ, Canton JH, Eijs YA, Everts JW, Janssen PJCM, v. Koten-Vermeulen JEM, Polder MD, Posthumus R, de Stoppelaar JM (1994) Towards integrated environmental quality objectives for several compounds with a potential for secondary poisoning: Underlying data. Annex to Report no. 679101 012, National Institute of Public Health and Environmental Protection, Bilthoven
Verdonck FAM, Aldenberg T, Jaworska J, Vanrolleghem PA (2003) Limitations of current risk characterization methods in probabilistic environmental risk assessment. Environ Toxicol Chem 22:2209–2213
Walse SS, Shimizu KD, Ferry JL (2002) Surface-catalyzed transformations of aqueous endosulfan. Environ Sci Technol 36:4846–4853
Walse SS, Scott GI, Ferry JL (2003) Stereoselective degradation of aqueous endosulfan in modular estuarine mesocosms: Formation of endosulfan gamma-hydroxycarboxylate. J Environ Monit 5:373–379
Wan MT, Kuo JN, Buday C, Schroeder G, Van Aggelen G, Pasternak J (2005) Toxicity of alpha-, beta-, (alpha plus beta)-endosulfan and their formulated and degradation products to Daphnia magna, Hyalella azteca, Oncorhynchus mykiss, Oncorhynchus kisutch, and biological implications in streams. Environ Toxicol Chem 24:1146–1154
Wauchope RD, Buttler TM, Hornsby AG, Augustijn-Beckers PWM, Burt JP (1992) The SCS/ARS/CES pesticide properties database for environmental decision-making. Rev Environ Contam Toxicol 123:1–164
Willey JB, Krone PH (2001) Effects of endosulfan and nonylphenol on the primordial germ cell population in pre-larval zebrafish embryos. Aquat Toxicol 54:113–123
Yao Y, Harner T, Blanchard P, Tuduri L, Waite D, Poissant L, Murphy C, Belzer W, Aulagnier F, Sverko E (2008) Pesticides in the atmosphere across Canadian agricultural regions. Environ Sci Technol 42:5931–5937
Zhao YA, Newman MC (2004) Shortcomings of the laboratory-derived median lethal concentration for predicting mortality in field populations: exposure duration and latent mortality. Environ Toxicol Chem 23:2147–2153
Zhou M, Li YC, Nkedi-Kizza P, O’Hair SK (2003) Endosulfan losses through runoff and leaching from calcareous gravelly or marl soils. Vadose Zone J 2:231–238
Acknowledgments
We would like to thank Adolfo Fernandez (Chemistry Department at FIU) for fish sampling and tissue analyses. Funding for this risk assessment project was provided by Cooperative Agreement number H5297-04-0133 with Everglades National Park. This is Southeast Environmental Research Center contribution number 470.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rand, G.M., Carriger, J.F., Gardinali, P.R. et al. Endosulfan and its metabolite, endosulfan sulfate, in freshwater ecosystems of South Florida: a probabilistic aquatic ecological risk assessment. Ecotoxicology 19, 879–900 (2010). https://doi.org/10.1007/s10646-010-0469-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-010-0469-0
Keywords
- Comprehensive Everglades Restoration Plan (CERP)
- Endosulfan
- α-endosulfan
- β-endosulfan
- Endosulfan sulfate
- Everglades National Park
- Organochlorines
- Probabilistic aquatic ecological risk assessment
- South Florida
- Internal effect concentration
- Criticial body residue
- Lethal body burden
- Species sensitivity distribution