Abstract
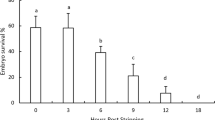
The quality of an oocyte can be defined by its potential to produce a normal and viable embryo. In Oncorhynchus mykiss (Walbaum), oocyte quality is highly variable under both natural and aquaculture conditions. To ensure the competitiveness and sustainability of rainbow trout farming, new tools are needed to evaluate oocyte potential. Considering that the abundance of certain maternal mRNAs incorporated during oogenesis can determine egg quality, the aim of the present study was to assess if significant differences existed in the abundances of the maternal mRNAs pou2 and zorba in low and high quality O. mykiss eggs. Analyses determined that survival until the end of gastrulation varied significantly between high and low quality egg batches, and significant correlations were established with posterior early development events. The abundance of pou2 and zorba transcripts varied between high and low quality groups, with higher relative expression recorded in the low quality group. Additionally, the abundances of these transcripts were significantly correlated with survival until blastopore closure, the earliest ontogenetic event evaluated as a quality attribute for this salmonid species. This correlation is supported by participations reported for both proteins in other teleost fish during embryonic development. These results form a basis for complementary studies and permit proposing maternal pou2 and zorba mRNA as potential markers for O. mykiss egg quality.



Similar content being viewed by others
References
Aas GH, Refstie T, Gjerde B (1991) Evaluation of milt quality of Atlantic Salmon. Aquaculture 95:125–132
Abrams EW, Mullins MC (2009) Early zebrafish development: It’s in the maternal genes. Curr Opin Genet Dev 19:396–403
Aegerter S, Jalabert B, Bobe J (2003) mRNA stockpile and egg quality in rainbow trout (Oncorhynchus mykiss). Fish Physiol Biochem 28:317–318
Aegerter S, Jalabert B, Bobe J (2004) Messenger RNA stockpile of cyclin B, insulin-like grow factor I, insulin-like grow factor II, insulin-like grow factor receptor Ib, and p53 in the rainbow trout oocyte in relation with development competence. Mol Reprod Dev 67:127–135
Aegerter S, Jalabert B, Bobe J (2005) Large scale real-time PCR analysis of mRNA abundance in rainbow trout eggs in relationship with egg quality and post-ovulatory ageing. Mol Reprod Dev 72:377–385
Andeol Y (1994) Early transcription in different animal species: implications for transition from maternal to zygotic control of development. Roux arch. Dev Biol 204:3–10
Babiak I, Glogowski J, Luczynski M, Goryczko K, Dobosz S, Kuzminski H (1998) The effect of individual male potency on fertilization ability of fresh and cryopreserved milt of rainbow trout, Oncorhynchus mykiss (Walbaum). Aquac Res 29:337–340
Bally-Cuif L, Schatz WJ, Ho RK (1998) Characterization of the zebra-fish Orb/CPEB-related RNA binding protein and localization of maternal components in the zebrafish oocyte. Mech Develop 77:31–47
Bellaiche J, Lareyre JJ, Cauty C, Yano A, Allemand I, LeGac F. (2014) Spermatogonial stem cell quest: nanos2, marker of a subpopulation of undifferentiated a spermatogonia in trout testis. Biol Reprod 90(4):79, 1–14
Bobe J (2015) Egg quality in fish: present and future challenges. Animals. Frontiers 5(1):66–72
Bobe J, Labbé C (2010) Egg and sperm quality in fish. Gen Comp Endocr 165:535–548
Bonnet E, Montfort J, Esquerre D, Hugot K, Fostier A, Bobe J (2007a) Effect of photoperiod manipulation on rainbow trout (Oncorhynchus mykiss) egg quality: a genomic study. Aquaculture 268:13–22
Bonnet E, Fostier A, Bobe J (2007b) Microarray-based analysis of fish egg quality after natural or controlled ovulation. BMC Genomics 8
Bromage NR, Randall JJ, Thrush M, Davies B, Springate J, Duston J, Barker G (1992) Broodstock management, fecundity, egg quality and the timing of egg production in the rainbow trout (Oncorhynchus mykiss). Aquaculture 100:141–166
Brooks S, Tyler CR, Sumpter JP (1997) Egg quality in fish: what makes a good egg? Rev Fish Biol Fisher 7:387–416
Christerson LB, McKearin DM (1994) Orb is required for anteroposterior and dorsoventral patterning during Drosophila oogenesis. Gen. Dev 8(5):614–628
Craik JCA, Harvey SM (1984) Egg quality in rainbow trout. The relation between egg viability, selected aspects of eggs composition, and time of stripping. Aquaculture 40:115–134
Dosch R, Wagner DS, Mintzer KA, Runke G, Wiemelt AP, Mullins MC (2004) Maternal control of vertebrate development before the midblastula transition: mutants from the zebrafish I. Dev Cell 6:771–780
Finch E, Cruz C, Sloman K, Kudoh T (2009) Heterochrony in the germ ring closure and tail bud formation in embryonic development of rainbow trout (Oncorhynchus mykiss). JExpZool (Mol.Dev. Ecol.) 314B:187–195
Gilbert, S. (2010) Developmental Biology. Sinauer Associates, Inc. Sunderland, MA
Gile SR, Ferguson MM (1995) Factors affecting male potency in pooled gamete crosses of rainbow trout, Oncorhynchus mykiss. Envir Biol Fish 42:267–275
Hake LE, Richter JD (1997) Translational regulation of maternal mRNA. Biochim Biophys Acta 1332:M31–M38
Hashimoto Y, Maegawa S, Nagai T, Yamaha E, Suzuki H, Yasuda K, Inoue K (2004) Localized maternal factors are requerid for zebrafish germ cell formation. Dev Biol 268:152–161
Kelley RL (1993) Initial organization of the Drosophila dorsoventral axis depends on an RNA-binding protein encoded by the squid gene. Gen Dev 6:948–960
Khan A, Nakamoto A, Okamoto S, Tai M, Nakayama Y, Kobayashi K, Kawamura A, Takeda H, Yamasu K (2012) Pou2, a class V POU-type transcription factor in zebrafish, regulates dorsoventral patterning and convergent extension movement at different blastula stages. Mech Develop 129(9–12):219–235. doi:10.1016/j.mod.2012.07.007
Kjorsvik E, Pitman K, Pavlov D (2004) From fertilization to the end of metamorphosis. Functional Development. In: Moksness, Kjorsvik, Olsen (eds) Culture of Cold-Water Marine Fish Blackwell Publishing Oxford pp 204–278
Kjørsvik E, Mangor-Jensen A, Holmefjord I (1990) Egg quality in fishes. Adv Mar Biol 26:71–113
Kunz, YW (2004) Developmental Biology of Teleost Fishes, Springer, Dublin
Lantz V, Ambrosio L, Schedl P (1992) The Drosophila orb gene is predicted to encode sex-specific germline RNA-binding proteins and has localized transcripts in ovaries and early embryos. Development 115(1):75–88
Liu R, Li M, Li Z, Hong N, Xu H, Hong Y (2015) Medaka Oct4 is essential for pluripotency in blastula formation and ES cell derivation. Stem Cell Rev and Rep 11:11–23
Manjeshwar S, Branam DE, Lerner MR, Brackett DJ, Jupe ER (2003) Tumor suppression by the prohibitin gene 3’untranslated region RNA in human breast cancer. Cancer Res 63(17):5251–5256
Marandel L, Labbe C, Bobe J, Jammes H, Lareyre JJ, Le Bail PY (2013) Do not put all teleosts in one net: focus on the sox2 and pou2 genes. Comp BiochemPhysiol B Biochem MolBiol 164(2):69–79
McKearin D, Christerson L (1994) Molecular genetics of the early stages of germ cell differentiation during Drosophila oogenesis. Ciba F Symp 182:210–219
Nagler JJ, Parsons JE, Cloud JG (2000) Single pair mating indicates maternal effects on embryo survival in rainbow trout, Oncorhynchus mykiss. Aquaculture 184:177–183
Norvell A, Debec A, Finch D, Gibson L, Thoma B (2005) Squid is required for efficient posterior localization of oskar mRNA during Drosophila oogenesis. Dev Genes Evol 215(7):340–349
O’Connell ML, Cavallo WC Jr, Firnberg M (2014) The expression of CPEB proteins is sequentially regulated during zebrafish oogenesis and embryogenesis. Mol Reprod Dev 81(4):376–387
Ramachandra RK, Salem M, Gahr S, Rexroad CE III, Yao J (2008) Cloning and characterization of microRNAs from rainbow trout (Oncorhynchus mykiss): their expression during early embryonic development. BMC Dev Biol 8
Reim G, Brand M (2006) Maternal control of vertebrate dorsoventral axis formation and epiboly by the POU domain Spg/Pou2/Oct4. Development 133:2757–2770
Richter JD (2007) CPEB: a life in translation. Trends Bio Chem Sci 32(6):279–285
Ridelman J, Hardy R, Brannon E (1984) The effect of short-term starvation on ovarian development and egg viability in rainbow trout. Aquaculture 37:133–140
Roberts W, White G (1992) Effects of angler wading on survival of trout eggs and pre-emergent fry. North Am J Fish Mana 12:450–459
Schier AF (2001) Axis formation and patterning in zebrafish. Curr Opin Genet Dev 11(4):393–404
Schier AF (2007) The maternal-zygotic transition: death and birth of RNAs. Science 316:–406
Shelton J L (1994) Trout production. Cooperative extension service, The University of Georgia College of Agricultural and Environmental Sciences. Aquaculture Technical Series: 1–15.
Skjærven KH, Olsvik PA, Finn RN, Holen E, Hamre K (2011) Ontogenetic expression of maternal and zygotic genes in Atlantic cod embryos under ambient and thermally stressed conditions. Comp Biochem. Phys A 159(2):196–205
Springate JRC, Bromage NR, Elliott JAK, Hudson DL (1984) The timing of ovulation and stripping and their effects on the rates of fertilization and survival to eyeing, hatch and swim-up in the rainbow trout (Salmo gairdneri) R. Aquaculture 43:313–322
Stebbins-Boaz B, Hake LE, Richter JD (1996) CPEB controls the cytoplasmic polyadenylation of cyclin, cdk2, and c-Mos mRNAs and is necessary for oocyte maturation in Xenopus. EMBO J 15:2582–2592
Steeger H, Freitag J, Michl S, Wiemer M, Paul R (2001) Effects of UV-B radiation on embryonic, larval and juvenile stages of north Seaplaice (Pleuronectes platessa) under simulated ozone-hole conditions. Helgoland Mar Res 55:56–66
Strahle U, Jesuthasan S (1993) Ultraviolet irradiation impairs epibolyin zebrafish embryos: evidence for a microtubule-dependent mechanismof epiboly. Development 119:909–919
Sullivan C, Chapman R, Reading B, Anderson P (2015) Transcriptomics of mRNA and egg quality in farmed fish: some recent developments and future directions. Gen Comp Endocr 221:23–30
Suzuki H, Tsukahara T, Inoue K (2009) Localization of c-mos mRNA around the animal pole in the zebrafish oocyte with Zor-1/Zorba. BioSci Trends 3(3):96–104
Takeda H, Matsuzaki T, Oki T, Miyagawa T, Amanuma H (1994) A novel POU domain gene, zebrafish pou2: expression and roles of two alternatively spliced twin products in early development. Genes Dev 8:45–59
Tata JR (1986) Coordinated asembly of the developing egg. Bio Essays 4:197–200
Wagner DS, Dosch R, Mintzer KA, Wiemelt AP, Mullins MC (2004) Maternal control of development at the midblastula transition and beyond: mutants from the zebrafish II. Dev Cell 6:781–790
Yang BY, Green M, Chen TT (1999) Early embryonic expression of the growth hormone family protein genes in the developing rainbow trout, Oncorhynchus mykiss. Mol Reprod Dev 53:127–134
Acknowledgments
This research was funded by the COPAS-Sur Austral Program (PFB-31/2007) awarded by the Universidad de Concepción, Chile; by Project 213.114.001-1AP awarded by the Universidad de Concepción, Chile, and by the projects INNOVA 12.178 (R. Aliaga), INNOVA Bio Bio 10-CHS2-690 F11 (D. Bravo), and INNOVA Bio Bio 102 (J.P. Alvarez). We also thank Ricardo Quiroz and Pablo Baldebenito from Salmones Pangue for access to the sampling facilities and aid in each of the experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cruzat, F., Bravo, D., Alvarez, J. et al. Abundance of the maternal mRNAs Pou2 and Zorba and their relation to events in the embryonic development of Oncorhynchus mykiss (Walbum). Environ Biol Fish 99, 845–855 (2016). https://doi.org/10.1007/s10641-016-0525-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-016-0525-6