Abstract
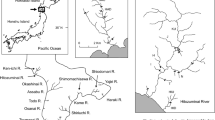
To increase the quantity of anadromous and freshwater fish resources and conserve natural populations effectively, it is important to identify conservation units and population structures, as well as to ensure sufficient genetic variability within populations. Here, we used 12 microsatellite loci to evaluate the population structure, effective population size, and bottlenecks in seven anadromous white-spotted char (Salvelinus leucomaenis leucomaenis) populations inhabiting the rivers of Hokkaido Island, Japan. Low migration rates were detected among populations, with significant genetic differentiation being observed, suggesting high homing rates. In addition, isolation-by-distance was observed among the evaluated populations, indicating that the populations in this region are at equilibrium between migration and drift. We identified a genetic bottleneck footprint in all seven of the analyzed white-spotted char populations by using the M ratio test. In contrast, heterozygote excess tests showed that all seven populations showed no signatures of population decline. This discrepancy may have been caused by differences in statistical power among tests. Alternatively, this discrepancy may be consistent with a strong founder effect during the late Pleistocene, followed by a subsequent low migration rate among populations. In conclusion, future conservation genetic management strategies should ensure that anadromous white-spotted char populations successfully exhibit homing behavior in the rivers of Hokkaido Island, Japan.




Similar content being viewed by others
References
Alo D, Turner TF (2005) Effects of habitat fragmentation on effective population size in the endangered Rio Grande silvery minnow. Conserv Biol 19:1138–1148
Angers B, Bernatchez L, Angers A, Desgroseillers L (1995) Specific microsatellite loci for brook charr (Salvelinus fontinalis Mitchill) reveals strong population subdivision on microgeographic scale. J Fish Biol 47:177–185
Antunes A, Faria R, Johnson WE, Guyomard R, Alexandrino P (2006) Life on the edge: the long-term persistence and contrasting spatial genetic structure of distinct brown trout life histories at their ecological limits. J Hered 97:193–205
Armstrong RH, Morrow JE (1980) The Dolly Varden charr. In: Balon EK (ed) Charrs: salmonid fishes of the genus Salvelinus. Dr W Junk b v, Publishers, The Hague, pp. 99–140
Asahida T, Kobayashi T, Saitoh K, Nakayama I (1996) Tissue preservation and total DNA extraction from fish stored at ambient temperature using buffers containing high concentration of urea. Fish Sci 62:727–730
Ayala JF (1965) Evolution of fitness in experimental populations of Drosophila Serrata. Science 150:903–905
Bams RA (1976) Survival and propensity for homing as affected by presence or absence of locally adapted paternal genes in two transplanted populations of pink salmon (Oncorhynchus gorbuscha). J Fish Res Board Can 33:2716–2725
Beerli P, Felsenstein J (1999) Maximum-likelihood estimation of migration rates and effective population numbers in two populations using a coalescent approach. Genetics 152:763–773
Beerli P, Felsenstein J (2001) Maximum likelihood estimation of a migration matrix and effective population sizes in n subpopulations by using a coalescent approach. Proc Natl Acad Sci U S A 98:4563–4568
Bernatchez L, Dempson JB, Martin S (1998) Microsatellite gene diversity analysis in anadromous Arctic charr, Salvelinus alpinus, from Labrador, Canada. Can J Fish Aquat Sci 55:1264–1272
Carvalho GR (1993) Evolutionary aspects of fish distribution: genetic variability and adaptation. J Fish Biol 43(Suppl. A):53–73
Castric V, Bernatchez L (2003) The rise and fall of isolation by distance in anadromous brook charr (Salvelinus fontinalis Mitchill). Genetics 165:983–996
El Mousadik A, Petit RJ (1996) High level of genetic differentiation for allelic richness among populations of the argan tree [Argania spinosa (L.) Skeels] endemic to Morocco. Theor Appl Genet 92:832–839
Excoffier L, Lischer HEL (2010) Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resour 10:564–567
Ferguson A (2004) The importance of identifying conservation units: Brown trout and pollan biodiversity in Ireland. P Roy Irish Acad 104B:33–41
Frankham R (1996) Relationship of genetic variation to population size in wildlife. Conserv Biol 10:1500–1508
Frankham R, Ballou JD, Briscoe DA (2002) Introduction to conservation genetics. Cambridge University press, Cambridge
Franklin IR (1980) Evolutionary change in small populations. In: Soule M, Wilcox B (eds) Conservation Biology: an Evolutionary–Ecological Perspective. Sinauer, Sunderland, pp. 135–149
Garza JC, Williamson EG (2001) Detection of reduction in population size using data from microsatellite loci. Mol Ecol 10:305–318
Goudet J (1995) FSTAT (vers.1.2): a computer program to calculate F-statistics. J Hered 86:485–486
Goudet J (2001) FSTAT, a program to estimate and test gene diversities and fixation indices (version 2.9.3). http://www2.unil.ch/popgen/softwares/fstat.htm. Accessed 5 May 2015
Groombridge B (1992) Centres of species diversity. In: Global biodiversity, Status of the Earth’s living resources, London, United Kingdom
Habel JC, Husemann M, Schmitt T, Dapporto L, Vandewoestijne S (2012) A forest butterfly in Sahara desert oases: isolation does not matter. J Hered 104:234–247
Hasselman DJ, Limburg KE (2012) Alosine restoration in the twenty-first century: challenging the status quo. Marine and Coastal Fisheries 4:174–187
Heath DD, Busch C, Kelly J, Atagi DY (2002) Temporal change in genetic structure and effective population size in steelhead trout (Oncorhynchus mykiss). Mol Ecol 11:197–214
Hendry AP, Castric V, Kinnison MT, Quinn TP (2004) The Evolution of Philopatry and Dispersal: Homing vs. Straying in Salmonids. In: Hendry AP, Stearns SC (eds) Evolution Illuminated: Salmon and Their Relatives. Oxford University Press, New York, pp. 52–91
Hoffman EA, Blouin MS (2004) Historical data refute recent range contraction as cause of low genetic diversity in isolated frog populations. Mol Ecol 13:71–276
Hosoya K (1993) Salmonidae. In: Nakabo T (ed) Fishes of Japan. Tokai University Press, Tokyo, pp. 256–261 (in Japanese)
Inamura A, Nakamura M (1962) Distribution and differentiation of Japanese char. Rep Res Inst Nat Resources 58-59:64–80 (in Japanese)
Jensen JL, Bohonak AJ, Kelley ST (2005) Isolation by distance, web service. BMC Genet 6:13 http://ibdws.sdsu.edu/. Accessed 5 May 2015
Johnson L (1980) The Arctic charr, Salvelinus alpinus. In: Balon EK (ed) Charrs – salmonid fishes of the genus Salvelinus. Dr W Junk b v, Publishers, The Hague, pp. 5–98
Jorde PE, Ryman N (1996) Demographic genetics of brown trout (Salmo trutta) and estimation of effective population size from temporal change in allele frequencies. Genetics 143:1369–1381
Kaeriyama M, Ueda H (1998) Life history strategy and migration pattern of juvenile sockeye (Oncorhynchus nerka) and chum salmon (O. keta) in Japan: a review. N Pac Anad Fish Comm Bull 1:163–171
Keller LF, Waller DM (2002) Inbreeding effects in wild populations. Trends Ecol Evol 17:230–241
Kikko T, Kai Y, Nakayama K (2009) Relationships among tributary length, census population size and genetic variability of white-spotted charr (Salvelinus leucomaenis) in the Lake Biwa water system. Ichthyol Res 56:100–104
Kirkpatrick M, Barton NH (1997) Evolution of a species’ range. Am Nat 150:1–23
Kishi D, Takayama H, Kato H, Fukushlma M (2003) Riverine fish fauna in the Hidaka region, Hokkaido, Res Bull Hokkaido Univ For 60: 1-18 (in Japanese)
Kitanishi S, Yamamoto T, Koizumi I, Dunham JB, Higashi S (2012) Fine scale relationships between sex, life history, and dispersal of masu salmon. Ecol Evol 2:920–929
Koizumi I, Yamamoto S, Maekawa K (2006) Decomposed pairwise regression analysis of genetic and geographic distances reveals a metapopulation structure of stream-dwelling Dolly Varden charr. Mol Ecol 15:3175–3189
Lacy RC (1987) Loss of genetic diversity from managed populations: interacting effects of drift, mutation, immigration, selection, and population subdivision. Conserv Biol 1:143–158
Laikre L, Jorde PE, Ryman N (1998) Temporal change of mitochondrial DNA haplotype frequencies and female effective size in a brown trout (Salmo trutta) population. Evolution 52:910–915
Leary RF, Allendorf FW, Sage GK (1995) Hybridization and introgression between introduced and native fish. Am Fish Soc Symp 15:91–101
Lenormand T (2002) Gene flow and the limits to natural selection. Trends Ecol Evol 17:183–189
Lowe WH, Allenndorf FW (2010) What can genetics tell us about population connectivity? Mol Ecol 19:3038–3051
Mantel N (1967) The detection of disease clustering and a generalized regression approach. Cancer Res 27:209–220
Maruyama T, Fuerst PA (1985) Population bottlenecks and nonequilibrium models in population genetics. II Number of alleles in a small population that was formed by a recent bottleneck. Genetics 111:675–689
Mills LS, Schwartz MK, Tallmon DA, Lair KP (2003) Measuring and interpreting connectivity for mammals in coniferous forests. In: Zabel CJ, Anthony RG (eds) Mammal community dynamics: management and conservation in the coniferous forests of western North America. Cambridge University Press, Cambridge, pp. 587–613
Mitton JB, Grant MC (1984) Associations among protein heterozygosity, growth rate, and developmental homeostasis. Annu Rev Ecol Syst 15:479–499
Moore JS, Harris LN, Tallman RF, Taylor EB (2013) The interplay between dispersal and gene flow in anadromous Arctic char (Salvelinus alpinus): implications for potential for local adaptation. Can J Fish Aquat Sci 70:1327–1338
Morita K, Yamamoto S (2002) Effects of habitat fragmentation by damming on the persistence of stream-dwelling charr populations. Conserv Biol 16:1318–1323
Morita K, Morita SH, Nagasawa T, Kuroki M (2013) Migratory patterns of anadromous white-spotted charr Salvelinus leucomaenis in eastern Hokkaido, Japan: the solution to a mystery? J Ichthyol 53:809–819
Moritz C (1994) Difining ‘Evolutionarily significant units’ for conservation. Trends Ecol Evol 9:373–375
Nakajima M, Fujio Y (1995) Genetic differentiation among local populations of Japanese char Salvelinus leucomaenis. Fish Sci 61:11–15
Nakamura T (1999) Comparison of physical characteristics of spawning redds between the fluvial Japanese charr Salvelinus leucomaenis and the masu salmon Oncorhynchus masou masou in headwaters of the Kinu River, central Japan. Nippon Suisan Gakkaishi 65:427–433 (in Japanese with English abstract)
Nei M, Maruyama T, Chakraborty R (1975) The bottleneck effect and genetic variability in populations. Evolution 29:1–10
Nei M, Tajima F, Tateno Y (1983) Accuracy of estimated phylogenetic trees from molecular data. J Mol Evol 19:153–170
Nordeng H (1971) Is the local orientation of anadromous fishes determined by pheromones? Nature 233:411–413
Nordeng H (2009) Char ecology. Natal homing in sympatric populations of anadromous Arctic char Salvelinus alpinus (L.): roles of pheromone recognition. Ecol Freshw Fish 18:41–51
Okazaki T (1986) Genetic variation and population structure in masu salmon Oncorhynchus masou of Japan. Bull Jpn Soc Sci Fish 52:1365–1376
Packer C, Pusey AE, Rowley H, Gilbert DA, Martenson J, O’Brien EJ (1991) Case study of a population bottleneck: Lions of the Ngorongoro Crater. Conserv Biol 5:219–230
Page RDM (1996) Tree View: an application to display phylogenetic trees on personal computers. Comput Appl Biosci 12:357–358
Palstra FP, Ruzzante DE (2010) A temporal perspective on population structure and gene flow in Atlantic salmon (Salmo salar) in Newfoundland. Can J Fish Aquat Sci 67:225–242
Parra D, García D, Méndez S, Cañón J, Dunner S (2010) High Mutation Rates in Canine tetranucleotide Microsatellites: Too Much Risk for Genetic Compatibility Purposes? Open Forensic Sci J 3:9–13
Pilgrim BL, Perry RC, Keefe DG, Perry EA, Marshall HD (2012) Microsatellite variation and genetic structure of brook trout (Salvelinus fontinalis) populations in Labrador and neighbouring Atlantic Canada: evidence for limited ongoing gene flow and dual routes of post-Wisconsinan colonization. Ecol Evol 2:885–898
Piry S, Luikart G, Cornuet J-M (1999) BOTTLENECK: A computer program for detecting recent detecting recent reductions in the effective populations size using allele frequency data. J Hered 90:502–503
Quattro JM, Vrijenhoek RC (1989) Fitness differences among remnant populations of the endangered Sonoran topminnow. Science 241:976–979
Quinn TP (1993) A review of homing and straying of wild and hatchery-produced salmon. Fish Res 18:29–44
Rice WR (1989) Analyzing tables of statistical tests. Evolution 43:223–225
Rieman BE, Allendorf FW (2001) Effective population size and genetic conservation criteria for bull trout. North Am J Fish Manage 21:756–764
Rousset F (1997) Genetic differentiation and estimation of gene flow from F-statistics under isolation by distance. Genetics 145:1219–1228
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstruction of phylogenetic trees. Mol Biol Evol 4:406–425
Shrimpton JM, Heath DD (2003) Census vs. effective population size in chinook salmon: large- and small-scale environmental perturbation effects. Mol Ecol 12:2571–2583
Slatkin M (1987) Gene flow and the geographical structure of natural populations. Science 236:787–792
Slatkin M (1993) Isolation by distance in equilibrium and non-equilibrium populations. Evolution 47:264–279
Stabell OB (1984) Homing and olfaction in salmonids — a critical review with special reference to the Atlantic salmon. Biol Rev 59:333–388
Swatdipong A, Primmer CR, Vasemägi A (2010) Historical and recent genetic bottlenecks in European grayling, Thymallus thymallus. Conserv Genet 11:279–292
Takehata Y, Kitagawa T (2010) Medaka (Oryzias latipes): genetic introgression resultingfrom artificial transplantation. Japan J Ichthyol 57:76–79 (in Japanese)
Takezaki N, Nei M, Tamura K (2010) POPTREE2: Software for constructing population trees from allele frequency data and computing other population statistics with Windows interface. Mol Biol Evol 27:747–752
Tallmon DA, Koyuk A, Luikart G, Beaumont M (2008) ONeSAMP: a program to estimate effective population size using approximate Bayesian computation. Mol Ecol Notes 8:299–301
Taniguchi N (2003) Genetic factor in broodstock management for seed production. Rev Fish Biol Fish 13:177–185
Taylor EB (1991) A review of local adaptation in Salmonidae, with particular reference to Pacific and Atlantic salmon. Aquaculture 98:185–207
Ueda H (2012) Physiological mechanisms of imprinting and homing migration in Pacific salmon Oncorhynchus spp. J Fish Biol 81:543–558
Williamson-Natesan EG (2005) Comparison of methods for detecting bottlenecks from mirosatellite loci. Conserv Genet 6:551–562
Wilson GA, Rannala B (2003) Bayesian inference of recent migration rates using multilocus genotypes. Genetics 163:1177–1191
Wilson KA, Magnuson JJ, Lodge DM, Hill AM, Kratz TK, Perry WL, Willis TV (2004) Dispersal in a stream-dwelling salmonid: Inferences from tagging and microsatellite studies. Conserv Genet 5:25–37
Wright S (1943) Isolation by distance. Genetics 28:114–138
Yamamoto S, Morita K, Kitano S, Watanabe K, Koizumi I, Maekawa K, Takamura K (2004a) Phylogeography of white-spotted charr (Salvelinus leucomaenis) inferred from mitochondrial DNA sequences. Zool Sci 21:229–240
Yamamoto S, Morita K, Koizumi I, Maekawa K (2004b) Genetic differentiation of white-spotted charr (Salvelinus leucomaenis) populations after habitat fragmentation: Spatial-temporal changes in gene frequencies. Conserv Genet 5:529–538
Yamaguchi K, Nakajima M, Taniguchi N (2008) Development of microsatellite markers in Japanese char Salvelinus leucomaenis and their applicability to closely related species. Fish Genet Breed Sci 38:123–130
Yamaguchi K, Nakajima M, Taniguchi N (2010) Loss of genetic variation and increased population differentiation in geographically peripheral populations of Japanese char Salvelinus leucomaenis. Aquaculture 308(suppl.1):S20–S27
Yamaguchi K, Saito M, Nakajima M (2015a) Identification and characterization of 12 tetranucleotide microsatellite markers in the white-spotted char Salvelinus leucomaenis. Conserv Genet Resour 7:497–499
Yamaguchi K, Nakajima M, Taniguchi N (2015b) Mitochondrial genetic evidence for recent population expansion of the white-spotted char (Salvelinus leucomaenis) without geographic patterns from Northern Japan to Central Honshu. Fish Genet Breed Sci 44:5–16
Acknowledgments
We thank Daisei Ando (Hokkaido Research Organization, Fisheries Research Department Salmon and Freshwater Fisheries Research Institute) and Hiromasa Yamaguchi (a fishing guide in Hokkaido) for assistance with sampling, and Mariko Miyazaki for technical assistance during our study. We also thank Daisuke Kishi (Gifu Prefecture Research Institute for Fisheries and Aquatic Environments) for his suggestion to collect data from the Mitsuishi River. In addition, we thank the anonymous reviewers for these helpful comments. This study was partly supported by JSPS Grants-in-Aid for Scientific Research, grant no.25252035.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yamaguchi, K., Nakajima, M. & Taniguchi, N. Population structure and conservation genetics of anadromous white-spotted char (Salvelinus leucomaenis) on Hokkaido Island: Detection of isolation-by-distance. Environ Biol Fish 99, 513–525 (2016). https://doi.org/10.1007/s10641-016-0494-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-016-0494-9