Summary
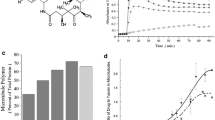
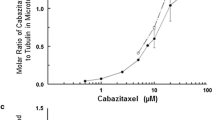
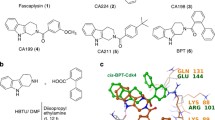
The avocado toxin (+)-R-persin (persin) is active at low micromolar concentrations against breast cancer cells and synergizes with the estrogen receptor modulator 4-hydroxytamoxifen. Previous studies in the estrogen receptor-positive breast cancer cell line MCF-7 indicate that persin acts as a microtubule-stabilizing agent. In the present study, we further characterize the properties of persin and several new synthetic analogues in human ovarian cancer cells. Persin and tetrahydropersin cause G2M cell cycle arrest and increase intracellular microtubule polymerization. One analog (4-nitrophenyl)-deshydroxypersin prevents cell proliferation and blocks cells in G1 of the cell cycle rather than G2M, suggesting an additional mode of action of these compounds independent of microtubules. Persin can synergize with other microtubule-stabilizing agents, and is active against cancer cells that overexpress the P-glycoprotein drug efflux pump. Evidence from Flutax-1 competition experiments suggests that while the persin binding site on β-tubulin overlaps the classical taxoid site where paclitaxel and epothilone bind, persin retains activity in cell lines with single amino acid mutations that affect these other taxoid site ligands. This implies the existence of a unique binding location for persin at the taxoid site.




Similar content being viewed by others
Abbreviations
- CI:
-
Combination index
- MDR:
-
Multidrug resistance
- MSA:
-
Microtubule-stabilizing agent
- P-gp:
-
P-glycoprotein
- T-persin:
-
Tetrahydropersin
References
Chang C-F, Isogai A, Kamikado T, Murakoshi S, Sakurai A, Tamura S (1975) Isolation and structure elucidation of growth inhibitors for silkworm larvae from avocado leaves. Agric Biol Chem 39:1167–1168
Oelrichs PB, JNg JC, Seawright AA, Ward W, Schäffeler L, MacLeod JK (1995) Isolation and identification of a compound from avocado (Persea americana) leaves which causes necrosis of the acinar epithelium of the lactating mammary gland and the myocardium. Nat Toxins 3:344–349
MacLeod JK, Schäffeler L (1995) A short enantioselective synthesis of a biologically active compound from Persea americana. J Nat Prod 58:1270–1273
Prusky D, Keen NT, Sims JJ, Midland SL (1982) Possible involvement of an antifungal diene in the latency of Colletotrichum gloeosporioides on unripe avocado fruits. Phytopathology 72:1578–1582
Rodriguez-Saona C, Millar JG, Maynard DF, Trumble JT (1998) Novel antifeedant and insecticidal compounds from avocado idioblast cell oil. J Chem Ecol 24:867–889
Carman RM, Duffield AR, Handley PN, Karoli T (2000) 2-Hydroxy-4-oxohenicosan-1-yl acetate. Its presence in avocado and its simple chemistry. Aust J Chem 53:191–194
Kingsbury JM (1964) Poisonous Plants of the United States and Canada. Prentice-Hall, Inc., Englewood Cliffs, 124
Menéndez JA, Barbacid MD, Montero S, Sevilla E, Escrich E, Solanas M, Cortes-Funes H, Colomer R (2001) Effects of gamma-linolenic acid and oleic acid on paclitaxel cytotoxicity in human breast cancer cells. Eur J Cancer 37:402–413
Butt AJ, Roberts CG, Seawright AA, Oelrichs PB, MacLeod JK, Liaw TYE, Kavallaris M, Somers-Edgar TJ, Lehrbach GM, Watts CK, Sutherland RL (2006) A novel plant toxin, persin, with in vivo activity in the mammary gland, induces Bim-dependent apoptosis in human breast cancer cells. Mol Cancer Ther 5:2300–2309
Roberts CG, Gurisik E, Biden TJ, Sutherland RL, Butt AJ (2007) Synergistic cytotoxicity between tamoxifen and the plant toxin persin in human breast cancer cells is dependent on Bim expression and mediated by modulation of ceramide metabolism. Mol Cancer Ther 6:2777–2785
McCloy RA, Shelley EJ, Roberts CG, Boslem E, Biden TJ, Nicholson RI, Gee JM, Sutherland RL, Musgrove EA, Burgess A, Butt AJ (2013) Role of endoplasmic reticulum stress induction by the plant toxin, persin, in overcoming resistance to the apoptotic effects of tamoxifen in human breast cancer cells. Brit J Cancer 109:3034–3041
Bonilla-Porras AR, Salazar-Ospina A, Jimenez-Del-Rio M, Pereañez-Jimenez A, Velez-Pardo C (2014) Pro-apoptotic effect of Persea americana var. Hass (avocado) on Jurkat lymphoblastic leukemia cells. Pharm Biol 52:458–465
Jordan MA, Wilson L (2004) Microtubules as a target for anticancer drugs. Nat Rev Cancer 4:253–265
Haldar S, Chintapalli J, Croce CM (1996) Taxol induces Bcl-2 phosphorylation and death of prostate cancer cells. Cancer Res 56:1253–1255
Kenny FS, Pinder SE, Ellis IO, Gee JM, Nicholson RL, Bryce RP, Robertson JF (2000) Gamma linolenic acid with tamoxifen as primary therapy in breast cancer. Int J Cancer 85:643–648
Puthalakath H, Huang DC, O'Reilly LA, King SM, Strasser A (1999) The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol Cell 3:287–296
Ding H, Chin YW, Kinghorn AD, D'Ambrosio SM (2007) Chemopreventive characteristics of avocado fruit. Semin Cancer Biol 17:386–394
Ding H, Han C, Guo D, Chin YW, Ding Y, Kinghorn AD, D'Ambrosio SM (2009) Selective induction of apoptosis of human oral cancer cell lines by avocado extracts via a ROS-mediated mechanism. Nutr Cancer 61:348–356
Paul R, Kulkarni P, Ganesh N (2011) Avocado fruit (Persea americana Mill) exhibits chemo-protective potentiality against cyclophosphamide induced genotoxicity in human lymphocyte culture. J Exp Ther Oncol 9:221–230
Lu H, Ouyang W, Huang C (2006) Inflammation, a key event in cancer development. Mol Cancer Res 4:221–233
Rodriguez-Saona C, Millar JG, Trumble JT (1998) Isolation, identification, and biological activity of isopersin, a new compound from avocado idioblast oil cells. J Nat Prod 61:1168–1170
Brooke DG, Shelley EJ, Roberts CG, Denny WA, Sutherland RL, Butt AJ (2011) Synthesis and in vitro evaluation of analogues of avocado-produced toxin (+)-(R)-persin in human breast cancer cells. Bioorg Med Chem 19:7033–7043
Prota AE, Bargsten K, Zurwerra D, Field JJ, Díaz JF, Altmann K-H, Steinmetz MO (2013) Molecular mechanism of action of microtubule-stabilizng agents. Science 339:587–590
Prota AE, Bargsten K, Northcote PT, Marsh M, Altmann K-H, Miller JH, Díaz JF, Steinmetz MO (2014) Structural basis of microtubule stabilization by laulimalide and peloruside A. Angew Chem Int Ed 53:1621–1625
West LM, Northcote PT, Battershill CN (2000) Peloruside A: a potent cytotoxic macrolide isolated from the New Zealand marine sponge Mycale sp. J Org Chem 65:445–449
Field JJ, Singh AJ, Kanakkanthara A, Halafihi T, Northcote PT, Miller JH (2009) Microtubule-stabilizing activity of zampanolide, a potent macrolide isolated from the Tongan marine sponge Cacospongia mycofijiensis. J Med Chem 52:7328–7332
Hood KA, West LM, Northcote PT, Berridge MV, Miller JH, Induction of apoptosis by the marine sponge (Mycale) metabolites, mycalamide A and pateamine. Apoptosis 6:207−219.
Giannakakou P, Sackett DL, Kang YK, Zhan Z, Buters JTM, Fojo T, Poruchynsky MS (1997) Paclitaxel-resistant human ovarian cancer cells have mutant β-tubulins that exhibit impaired paclitaxel-driven polymerization. J Biol Chem 272:17118–17125
Wilmes A, Bargh K, Kelly C, Northcote PT, Miller JH (2007) Peloruside A synergizes with other microtubule stabilizing agents in cultured cancer cell lines. Mol Pharm 4:269–280
Wilmes A, O’Sullivan D, Chan A, Chandrahasen C, Paterson I, Northcote PT, La Flamme AC, Miller JH (2011) Synergistic interactions between peloruside A and other microtubule-stabilizing and destabilizing agents in cultured human ovarian carcinoma cells and murine T cells. Cancer Chemother Pharmacol 68:117–126
Field JJ, Calvo E, Northcote PT, Miller JH, Altmann K-H, Díaz JF (2013) Methods for studying microtubule binding site interactions: Zampanolide as a covalent binding agent. In Wilson L and Correia JJ (eds) Methods in Cell Biology, Vol 115: Microtubules In Vitro, 2nd Ed, Academic Press, Burlington, Chapter 19, pp. 303−325
Lalande M (1990) A reversible arrest point in the late G1 phase of the mammalian cell cycle. Exp Cell Res 186:332–339
Kanakkanthara A, Wilmes A, O'Brate A, Escuin D, Chan A, Gjyrezi A, Crawford J, Rawson P, Kivell B, Northcote PT, Hamel E, Giannakakou P, Miller JH (2011) Peloruside- and laulimalide-resistant human ovarian carcinoma cells have βI-tubulin mutations and altered expression of βII- and βIII-tubulin isotypes. Mol Cancer Ther 10:1419–1429
Giannakakou P, Gussio R, Nogales E, Downing KH, Zaharevitz D, Bollbuck B, Poy G, Sackett D, Nicolaou KC, Fojo T (2000) A common pharmacophore for epothilone and taxanes: molecular basis for drug resistance conferred by tubulin mutations in human cancer cells. Proc Natl Acad Sci U S A 97:2904–2909
Gaitanos TN, Buey RM, Díaz JF, Northcote PT, Teesdale-Spittle P, Andreu JM, Miller JH (2004) Peloruside A does not bind to the taxoid site on β-tubulin and retains its activity in multidrug resistant cell lines. Cancer Res 64:5063–5067
Ding C, Zhang Y, Chen H, Yang Z, Wild C, Ye N, Ester CD, Xiong A, White MA, Shen Q, Zhou J (2013) Oridonin ring A-based diverse constructions of enone functionality: identification of novel dienone analogues effective for highly aggressive breast cancer by inducing apoptosis. J Med Chem 56:8814–8825
Phase 1 safety study of ACT-PFK-158, 2HCl in patients with advanced solid malignancies: https://clinicaltrials.gov/ct2/show/NCT02044861 Accessed 18 Dec 2015
Hashimura H, Ueda C, Kawabata J, Kasai T (2001) Acetyl-CoA carboxylase inhibitors from avocado (Persea americana Mill) fruits. Biosci Biotechnol Biochem 65:1656–1658
Ferlini C, Cicchillitti L, Raspaglio G, Bartollino S, Cimitan S, Bertucci C, Mozzetti S, Gallo D, Persico M, Fattorusso C, Campiani G, Scambia G (2009) Paclitaxel directly binds to Bcl-2 and functionally mimics activity of Nur77. Cancer Res 69:6906–6914
Ferlini C, Raspaglio G, Mozzetti S, Martinelli E, Ferrandina G, Gallo D, Riva A, Bombardelli E, Morazzoni P, Scambia G (2003) Bcl-2 downregulation is a novel mechanism of paclitaxel resistance. P. Am. Assoc Cancer Res 44:1104–1104
Menéndez JA, Ropero S, del Barbacid MM, Montero S, Solanas M, Escrich E, Cortés-Funes H, Colomer R (2002) Synergistic interaction between vinorelbine and gamma-linolenic acid in breast cancer cells. Breast Cancer Res Treat 72:203–219
Kowalski RJ, Giannakakou P, Gunasekera P, Longley RE, Day BW, Hamel E (1997) The microtubule-stabilizing agent discodermolide competitively inhibits the binding of paclitaxel (Taxol) to tubulin polymers, enhances tubulin nucleation reactions more potently than paclitaxel, and inhibits the growth of paclitaxel-resistant cells. Mol Pharmacol 52:613–622
He L, Yang C-PH, Horwitz SB (2001) Mutations in β-tubulin map to domains involved in regulation of microtubule stability in epothilone-resistant cell lines. Mol Cancer Ther 1:3–10
Martello LA, McDaid HM, Regl DL, Yang CP, Meng D, Pettus TR, Kaufman MD, Arimoto H, Danishefsky SJ, Smith AB 3rd, Horwitz SB (2000) Taxol and discodermolide represent a synergistic drug combination in human carcinoma cell lines. Clin Cancer Res 6:1978–1987
Giannakakou P (2000) Fojo T (2000) Discodermolide: just another microtubule- stabilizing agent? No! A lesson in synergy. Clin Cancer Res 6:1613–1615
Honore S, Kamath K, Braguer D, Horwitz SB, Wilson L, Briand C, Jordan MA (2004) Synergistic suppression of microtubule dynamics by discodermolide and paclitaxel in non-small cell lung carcinoma cells. Cancer Res 64:4957–4964
Acknowledgments
This work was supported by grants from the Cancer Society of New Zealand, Wellington Medical Research Foundation (JJF, AK, JHM), the Joy McNicoll Postgraduate Research Award in Biomedical Science (JJF), and Victoria University of Wellington (JJF, AK, JHM). We thank Dr Paraskevi Giannakkakou for the 1A9 β-tubulin mutant cell lines, and Dr Peter Northcote and Dr Jonathan Singh for the supply of the natural product peloruside A.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Jessica Field and Arun Kanakkanthara contributed equally to the paper.
Rights and permissions
About this article
Cite this article
Field, J.J., Kanakkanthara, A., Brooke, D.G. et al. Microtubule-stabilizing properties of the avocado-derived toxins (+)-(R)-persin and (+)-(R)-tetrahydropersin in cancer cells and activity of related synthetic analogs. Invest New Drugs 34, 277–289 (2016). https://doi.org/10.1007/s10637-016-0341-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-016-0341-z