Summary
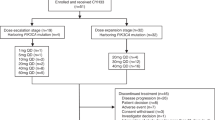
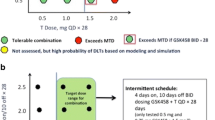
Purpose Combining agents that block both the VEGF and PI3K/AKT/mTOR pathways may be synergistic. We explored a novel dosing schedule to assess safety, toxicity and activity in patients with advanced solid tumors. Patients and Methods Patients with refractory solid tumors were enrolled in a modified 3 + 3 Phase I dose escalation study to determine dose limiting toxicities (DLTs) and the maximum tolerated dose (MTD) of a combination of everolimus (mTOR inhibitor) and pazopanib (tyrosine kinase inhibitor with anti-VEGF activity). An expansion cohort selected for patients with molecular alterations in the PI3K/AKT/mTOR pathway. Results Sixty-two patients were enrolled; median age was 60 years; 29 were women. The MTD was pazopanib 600 mg every other day (QOD) alternating with everolimus 10 mg PO QOD. DLTs were grade 3 thrombocytopenia and creatinine elevation. Most common toxicities of any grade were thrombocytopenia, transaminitis, leukopenia/neutropenia and lipid abnormalities. Among 52 patients evaluable for response, the clinical benefit rate (CBR) was 27 % (14/52) including four partial responses (PR), and 10 stable disease (SD) ≥6 months. 26 of 45 patients evaluated for molecular alterations had at least one alteration in the PI3K/AKT/mTOR pathway. CBR in patients with a matched alteration was 27 % (7/26) versus 26 % (5/19) for patients without an alteration (p = 0.764). However, 64% of those with CBR and molecular testing done for alteration in the PI3K/AKT/mTOR pathway were positive. Conclusion Combination treatment with pazopanib and everolimus was well tolerated and demonstrated activity in solid tumors. Further exploration of this combination and molecular correlation with treatment outcomes is warranted.

Similar content being viewed by others
References
Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350(23):2335–2342. doi:10.1056/NEJMoa032691
Hicklin DJ, Ellis LM (2005) Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 23(5):1011–1027. doi:10.1200/JCO.2005.06.081
Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA (2003) A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 349(5):427–434. doi:10.1056/NEJMoa021491
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285(21):1182–1186. doi:10.1056/NEJM197111182852108
Ferrara N (2005) The role of VEGF in the regulation of physiological and pathological angiogenesis. EXS 94:209–231
Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L, Dickler M, Overmoyer BA, Reimann JD, Sing AP, Langmuir V, Rugo HS (2005) Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol 23(4):792–799. doi:10.1200/JCO.2005.05.098
Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, Langer CJ, DeVore RF 3rd, Gaudreault J, Damico LA, Holmgren E, Kabbinavar F (2004) Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol 22(11):2184–2191. doi:10.1200/JCO.2004.11.022
Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, Sovak MA, Yi J, Nycum LR (2012) OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol 30(17):2039–2045. doi:10.1200/JCO.2012.42.0505
Juengel E, Engler J, Natsheh I, Jones J, Mickuckyte A, Hudak L, Jonas D, Blaheta RA (2009) Combining the receptor tyrosine kinase inhibitor AEE788 and the mammalian target of rapamycin (mTOR) inhibitor RAD001 strongly inhibits adhesion and growth of renal cell carcinoma cells. BMC Cancer 9:161. doi:10.1186/1471-2407-9-161
Park HS, Hong SK, Oh MM, Yoon CY, Jeong SJ, Byun SS, Cheon J, Lee SE, du Moon G (2014) Synergistic antitumor effect of NVP-BEZ235 and sunitinib on docetaxel-resistant human castration-resistant prostate cancer cells. Anticancer Res 34(7):3457–3468
LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA (2008) Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist Updat 11(1-2):32–50. doi:10.1016/j.drup.2007.11.003
Papadimitrakopoulou VA, Soria JC, Jappe A, Jehl V, Klimovsky J, Johnson BE (2012) Everolimus and erlotinib as second- or third-line therapy in patients with advanced non-small-cell lung cancer. J Thorac Oncol 7(10):1594–1601. doi:10.1097/Jto.0b013e3182614835
Nilsson MB, Zage PE, Zeng L, Xu L, Cascone T, Wu HK, Saigal B, Zweidler-McKay PA, Heymach JV (2010) Multiple receptor tyrosine kinases regulate HIF-1alpha and HIF-2alpha in normoxia and hypoxia in neuroblastoma: implications for antiangiogenic mechanisms of multikinase inhibitors. Oncogene 29(20):2938–2949. doi:10.1038/onc.2010.60
Cam H, Easton JB, High A, Houghton PJ (2010) mTORC1 signaling under hypoxic conditions is controlled by ATM-dependent phosphorylation of HIF-1alpha. Mol Cell 40(4):509–520. doi:10.1016/j.molcel.2010.10.030
Brugarolas J (2007) Renal-cell carcinoma–molecular pathways and therapies. N Engl J Med 356(2):185–187. doi:10.1056/NEJMe068263
Heng DY, Bukowski RM (2008) Anti-angiogenic targets in the treatment of advanced renal cell carcinoma. Curr Cancer Drug Targets 8(8):676–682
Meric-Bernstam F, Gonzalez-Angulo AM (2009) Targeting the mTOR signaling network for cancer therapy. J Clin Oncol 27(13):2278–2287. doi:10.1200/JCO.2008.20.0766
Bukowski RM (2010) Pazopanib: a multikinase inhibitor with activity in advanced renal cell carcinoma. Expert Rev Anticancer Ther 10(5):635–645. doi:10.1586/era.10.38
Gotink KJ, Verheul HM (2010) Anti-angiogenic tyrosine kinase inhibitors: what is their mechanism of action? Angiogenesis 13(1):1–14. doi:10.1007/s10456-009-9160-6
Mabuchi S, Altomare DA, Cheung M, Zhang L, Poulikakos PI, Hensley HH, Schilder RJ, Ozols RF, Testa JR (2007) RAD001 inhibits human ovarian cancer cell proliferation, enhances cisplatin-induced apoptosis, and prolongs survival in an ovarian cancer model. Clin Cancer Res 13(14):4261–4270. doi:10.1158/1078-0432.CCR-06-2770
Dormond O, Madsen JC, Briscoe DM (2007) The effects of mTOR-Akt interactions on anti-apoptotic signaling in vascular endothelial cells. J Biol Chem 282(32):23679–23686. doi:10.1074/jbc.M700563200
Patel PH, Senico PL, Curiel RE, Motzer RJ (2009) Phase I study combining treatment with temsirolimus and sunitinib malate in patients with advanced renal cell carcinoma. Clin Genitourin Cancer 7(1):24–27. doi:10.3816/CGC.2009.n.004
Desar IM, Timmer-Bonte JN, Burger DM, van der Graaf WT, van Herpen CM (2010) A phase I dose-escalation study to evaluate safety and tolerability of sorafenib combined with sirolimus in patients with advanced solid cancer. Br J Cancer 103(11):1637–1643. doi:10.1038/sj.bjc.6605777
Semrad TJ, Eddings C, Dutia MP, Christensen S, Lara PN Jr (2013) Phase I study of the combination of temsirolimus and pazopanib in advanced solid tumors. Anticancer Drugs 24(6):636–640. doi:10.1097/CAD.0b013e3283618b7b
Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, Hamid O, Schuchter L, Cebon J, Ibrahim N, Kudchadkar R, Burris HA 3rd, Falchook G, Algazi A, Lewis K, Long GV, Puzanov I, Lebowitz P, Singh A, Little S, Sun P, Allred A, Ouellet D, Kim KB, Patel K, Weber J (2012) Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 367(18):1694–1703. doi:10.1056/NEJMoa1210093
Thomas RK (2014) Overcoming drug resistance in ALK-rearranged lung cancer. N Engl J Med 370(13):1250–1251. doi:10.1056/NEJMe1316173
Miller VA, Hirsh V, Cadranel J, Chen YM, Park K, Kim SW, Zhou C, Su WC, Wang M, Sun Y, Heo DS, Crino L, Tan EH, Chao TY, Shahidi M, Cong XJ, Lorence RM, Yang JC (2012) Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol 13(5):528–538. doi:10.1016/S1470-2045(12)70087-6
Fishman MN, Srinivas S, Hauke RJ, Amato RJ, Esteves B, Cotreau MM, Strahs AL, Slichenmyer WJ, Bhargava P, Kabbinavar FF (2013) Phase Ib study of tivozanib (AV-951) in combination with temsirolimus in patients with renal cell carcinoma. Eur J Cancer 49(13):2841–2850. doi:10.1016/j.ejca.2013.04.019
Amato RJ, Flaherty AL, Stepankiw M (2012) Phase I trial of everolimus plus sorafenib for patients with advanced renal cell cancer. Clin Genitourin Cancer 10(1):26–31. doi:10.1016/j.clgc.2011.11.002
Harzstark AL, Small EJ, Weinberg VK, Sun J, Ryan CJ, Lin AM, Fong L, Brocks DR, Rosenberg JE (2011) A phase 1 study of everolimus and sorafenib for metastatic clear cell renal cell carcinoma. Cancer 117(18):4194–4200. doi:10.1002/cncr.25931
Chan JA, Mayer RJ, Jackson N, Malinowski P, Regan E, Kulke MH (2013) Phase I study of sorafenib in combination with everolimus (RAD001) in patients with advanced neuroendocrine tumors. Cancer Chemother Pharmacol 71(5):1241–1246. doi:10.1007/s00280-013-2118-9
Finn RS, Poon RT, Yau T, Klumpen HJ, Chen LT, Kang YK, Kim TY, Gomez-Martin C, Rodriguez-Lope C, Kunz T, Paquet T, Brandt U, Sellami D, Bruix J (2013) Phase I study investigating everolimus combined with sorafenib in patients with advanced hepatocellular carcinoma. J Hepatol 59(6):1271–1277. doi:10.1016/j.jhep.2013.07.029
Toffalorio F, Spitaleri G, Catania C, Dal Zotto L, Noberasco C, Delmonte A, Santarpia M, Vecchio F, Brunelli V, Rampinelli C, Barberis M, Fumagalli C, Zucchetti M, Zangarini M, Diena T, Danesi R, de Braud F, De Pas T (2014) Phase ib of sorafenib in combination with everolimus in patients with advanced solid tumors, selected on the basis of molecular targets. Oncologist 19(4):344–345. doi:10.1634/theoncologist.2013-0335
Wagle N, Grabiner BC, Van Allen EM, Hodis E, Jacobus S, Supko JG, Stewart M, Choueiri TK, Gandhi L, Cleary JM, Elfiky AA, Taplin ME, Stack EC, Signoretti S, Loda M, Shapiro GI, Sabatini DM, Lander ES, Gabriel SB, Kantoff PW, Garraway LA, Rosenberg JE (2014) Activating mTOR mutations in a patient with an extraordinary response on a phase I trial of everolimus and pazopanib. Cancer Discover 4(5):546–553. doi:10.1158/2159-8290.CD-13-0353
Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG, Tomassetti P, Pavel ME, Hoosen S, Haas T, Lincy J, Lebwohl D, Oberg K (2011) Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 364(6):514–523. doi:10.1056/NEJMoa1009290
Altorki N, Lane ME, Bauer T, Lee PC, Guarino MJ, Pass H, Felip E, Peylan-Ramu N, Gurpide A, Grannis FW, Mitchell JD, Tachdjian S, Swann RS, Huff A, Roychowdhury DF, Reeves A, Ottesen LH, Yankelevitz DF (2010) Phase II proof-of-concept study of pazopanib monotherapy in treatment-naive patients with stage I/II resectable non-small-cell lung cancer. J Clin Oncol 28(19):3131–3137. doi:10.1200/JCO.2009.23.9749
Baselga J, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun F, Beck JT, Ito Y, Yardley D, Deleu I, Perez A, Bachelot T, Vittori L, Xu Z, Mukhopadhyay P, Lebwohl D, Hortobagyi GN (2012) Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 366(6):520–529. doi:10.1056/NEJMoa1109653
Oza AM, Elit L, Tsao MS, Kamel-Reid S, Biagi J, Provencher DM, Gotlieb WH, Hoskins PJ, Ghatage P, Tonkin KS, Mackay HJ, Mazurka J, Sederias J, Ivy P, Dancey JE, Eisenhauer EA (2011) Phase II study of temsirolimus in women with recurrent or metastatic endometrial cancer: a trial of the NCIC Clinical Trials Group. J Clin Oncol 29(24):3278–3285. doi:10.1200/JCO.2010.34.1578
Alvarez EA, Brady WE, Walker JL, Rotmensch J, Zhou XC, Kendrick JE, Yamada SD, Schilder JM, Cohn DE, Harrison CR, Moore KN, Aghajanian C (2013) Phase II trial of combination bevacizumab and temsirolimus in the treatment of recurrent or persistent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol 129(1):22–27. doi:10.1016/j.ygyno.2012.12.022
Tinker AV, Ellard S, Welch S, Moens F, Allo G, Tsao MS, Squire J, Tu D, Eisenhauer EA, MacKay H (2013) Phase II study of temsirolimus (CCI-779) in women with recurrent, unresectable, locally advanced or metastatic carcinoma of the cervix. a trial of the NCIC Clinical Trials Group (NCIC CTG IND 199). Gynecol Oncol 130(2):269–274. doi:10.1016/j.ygyno.2013.05.008
Bauman JE, Arias-Pulido H, Lee SJ, Fekrazad MH, Ozawa H, Fertig E, Howard J, Bishop J, Wang H, Olson GT, Spafford MJ, Jones DV, Chung CH (2013) A phase II study of temsirolimus and erlotinib in patients with recurrent and/or metastatic, platinum-refractory head and neck squamous cell carcinoma. Oral Oncol 49(5):461–467. doi:10.1016/j.oraloncology.2012.12.016
McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Franklin RA, Montalto G, Cervello M, Libra M, Candido S, Malaponte G, Mazzarino MC, Fagone P, Nicoletti F, Basecke J, Mijatovic S, Maksimovic-Ivanic D, Milella M, Tafuri A, Chiarini F, Evangelisti C, Cocco L, Martelli AM (2012) Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascade inhibitors: how mutations can result in therapy resistance and how to overcome resistance. Oncotarget 3(10):1068–1111
Malone CF, Fromm JA, Maertens O, DeRaedt T, Ingraham R, Cichowski K (2014) Defining key signaling nodes and therapeutic biomarkers in NF1-mutant cancers. Cancer Discover. doi:10.1158/2159-8290.CD-14-0159
Janku F, Wheler JJ, Westin SN, Moulder SL, Naing A, Tsimberidou AM, Fu S, Falchook GS, Hong DS, Garrido-Laguna I, Luthra R, Lee JJ, Lu KH, Kurzrock R (2012) PI3K/AKT/mTOR inhibitors in patients with breast and gynecologic malignancies harboring PIK3CA mutations. J Clin Oncol : Off J Am Soc Clin Oncol 30(8):777–782. doi:10.1200/JCO.2011.36.1196
Contreras CM, Akbay EA, Gallardo TD, Haynie JM, Sharma S, Tagao O, Bardeesy N, Takahashi M, Settleman J, Wong KK, Castrillon DH (2010) Lkb1 inactivation is sufficient to drive endometrial cancers that are aggressive yet highly responsive to mTOR inhibitor monotherapy. Dis Model Mech 3(3-4):181–193. doi:10.1242/dmm.004440
Zhou W, Marcus AI, Vertino PM (2013) Dysregulation of mTOR activity through LKB1 inactivation. Chin J Cancer 32(8):427–433. doi:10.5732/cjc.013.10086
Bader AG, Kang S, Vogt PK (2006) Cancer-specific mutations in PIK3CA are oncogenic in vivo. Proc Natl Acad Sci U S A 103(5):1475–1479. doi:10.1073/pnas.0510857103
Meyer DS, Koren S, Leroy C, Brinkhaus H, Muller U, Klebba I, Muller M, Cardiff RD, Bentires-Alj M (2013) Expression of PIK3CA mutant E545K in the mammary gland induces heterogeneous tumors but is less potent than mutant H1047R. Oncogenesis 2, e74. doi:10.1038/oncsis.2013.38
Janku F, Hong DS, Fu S, Piha-Paul SA, Naing A, Falchook GS, Tsimberidou AM, Stepanek VM, Moulder SL, Lee JJ, Luthra R, Zinner RG, Broaddus RR, Wheler JJ, Kurzrock R (2014) Assessing PIK3CA and PTEN in early-phase trials with PI3K/AKT/mTOR inhibitors. Cell Rep 6(2):377–387. doi:10.1016/j.celrep.2013.12.035
Janku F, Wheler JJ, Naing A, Falchook GS, Hong DS, Stepanek VM, Fu S, Piha-Paul SA, Lee JJ, Luthra R, Tsimberidou AM, Kurzrock R (2013) PIK3CA mutation H1047R is associated with response to PI3K/AKT/mTOR signaling pathway inhibitors in early-phase clinical trials. Cancer Res 73(1):276–284. doi:10.1158/0008-5472.CAN-12-1726
Nault JC, Mallet M, Pilati C, Calderaro J, Bioulac-Sage P, Laurent C, Laurent A, Cherqui D, Balabaud C, Zucman-Rossi J (2013) High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat Commun 4:2218. doi:10.1038/ncomms3218
Acknowledgments
Financial support
This study was approved and funded by the National Comprehensive Cancer Network (NCCN) Oncology Research Program from general research support provided by GlaxoSmithKline and The University of Texas MD Anderson Cancer Center Support Grant P30 CA016672 (F. Meric-Bernstam, J. Wheler).
Compliance with ethical standards
ᅟ
Disclosure of potential conflict of interest
Filip Janku received research funding from Novartis. Dr. Falchook received major commercial research grant from GlaxoSmithKline and Novartis and travel reimbursement from GlaxoSmithKline. All remaining authors declare no conflicts of interest.
Research involving human participants
The study was reviewed and approved by the MD Anderson Institutional Review Board. All procedures were performed in accordance with the ethical standards of the institutional and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all patients before study-related procedures were started.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
(DOC 68 kb)
Rights and permissions
About this article
Cite this article
Rodrigues, H.V., Ke, D., Lim, J. et al. Phase I combination of pazopanib and everolimus in PIK3CA mutation positive/PTEN loss patients with advanced solid tumors refractory to standard therapy. Invest New Drugs 33, 700–709 (2015). https://doi.org/10.1007/s10637-015-0238-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-015-0238-2