Summary
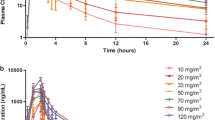
Background We determined the safety, pharmacokinetics, pharmacodynamics, and antitumour activity of abexinostat in B-cell lymphoma or chronic lymphocytic leukaemia. Patients and methods Thirty-five patients received oral abexinostat 30, 45, or 60 mg/m2 bid in a 3 + 3 design in three 21-day schedules: 14 days on treatment in schedule 1 (D1–14); 10 days in schedule 2 (D1–5 and D8–12); and 12 days in schedule 3 (D1–4, D8–11, and D15–18). Safety, tumour response, plasma concentration, and histone H3 acetylation were measured. Results Two dose-limiting toxicities occurred in each schedule (one grade 3 febrile neutropenia; five grade 4 thrombocytopenia) at 60 mg/m2 bid (maximal tolerated dose). The recommended dose was 45 mg/m2 bid; schedule 1 was considered optimal. Non-haematological drug-related toxicities included grade 1 or 2 diarrhoea (43 %), nausea (23 %), and vomiting (11 %); haematological toxicities included thrombocytopenia (31 % grade 3, and 26 % grade 4), which remained manageable and reversible on withdrawal. Of 29 evaluable patients, there were 2 complete and 6 partial responses; median duration of response was 14.6 months (range 3–16.5 months) (1 cycle is equivalent to 0.75 months). There was no evidence for nonlinear pharmacokinetics. There was a correlation between dose and histone acetylation. Conclusion Abexinostat has manageable toxicity and induced some durable complete and partial responses in B-cell lymphoma or chronic lymphocytic leukaemia. Our results suggest most favourable responses in patients with follicular lymphoma, though further research would be needed to confirm this finding.






Similar content being viewed by others
References
Bolden JE, Peart MJ, Johnstone RW (2006) Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov 5:769–784
Ververis K, Hiong A, Karagiannis TC, Licciardi PV (2013) Histone deacetylase inhibitors (HDACIs): multitargeted anticancer agents. Biologics 7:47–60
Glass E, Viale PH (2013) Histone deacetylase inhibitors: novel agents in cancer treatment. Clin J Oncol Nurs 17:34–40
Richon VM, Garcia-Vargas J, Hardwick JS (2009) Development of vorinostat: current applications and future perspectives for cancer therapy. Cancer Lett 280:201–210
Mann BS, Johnson JR, Cohen MH, Justice R, Pazdur R (2007) FDA approval summary: vorinostat for treatment of advanced primary cutaneous T-cell lymphoma. Oncologist 12:1247–1252
Coiffier B, Pro B, Prince HM, Foss F, Sokol L, Greenwood M et al (2012) Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol 30:631–636
Watanabe T (2010) Investigational histone deacetylase inhibitors for non-Hodgkin lymphomas. Expert Opin Investig Drugs 19:1113–1127
Buglio D, Younes A (2010) Histone deacetylase inhibitors in Hodgkin lymphoma. Invest New Drugs 28(Suppl 1):S21–S27
Veliz M, Pinilla-Ibarz J (2012) Treatment of relapsed or refractory chronic lymphocytic leukemia. Cancer Control 19:37–53
Lopez G, Liu J, Ren W, Wei W, Wang S, Lahat G et al (2009) Combining PCI-24781, a novel histone deacetylase inhibitor, with chemotherapy for the treatment of soft tissue sarcoma. Clin Cancer Res 15:3472–3483
Buggy JJ, Cao ZA, Bass KE, Verner E, Balasubramanian S, Liu L et al (2006) CRA-024781: a novel synthetic inhibitor of histone deacetylase enzymes with antitumor activity in vitro and in vivo. Mol Cancer Ther 5:1309–1317
Rivera-Del VN, Gao S, Miller CP, Fulbright J, Gonzales C, Sirisawad M et al (2010) PCI-24781, a novel hydroxamic Acid HDAC inhibitor, exerts cytotoxicity and histone alterations via caspase-8 and FADD in leukemia cells. Int J Cell Biol 207420
Yang C, Choy E, Hornicek FJ, Wood KB, Schwab JH, Liu X et al (2011) Histone deacetylase inhibitor (HDACI) PCI-24781 potentiates cytotoxic effects of doxorubicin in bone sarcoma cells. Cancer Chemother Pharmacol 67:439–446
Yang C, Choy E, Hornicek FJ, Wood KB, Schwab JH, Liu X et al (2011) Histone deacetylase inhibitor PCI-24781 enhances chemotherapy-induced apoptosis in multidrug-resistant sarcoma cell lines. Anticancer Res 31:1115–1123
National Cancer Institute. Common terminology criteria for adverse events. Version 3.0. NCI 2006; http://ctep.cancer.gov. Accessed 2-4-2013.
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET et al (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649–655
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ et al (2007) Revised response criteria for malignant lymphoma. J Clin Oncol 25:579–586
Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H et al (2008) Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the international workshop on chronic lymphocytic leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 111:5446–5456
Fouliard S, Robert R, Jacquet-Bescond A, du Rieu QC, Balasubramanian S, Loury D et al (2013) Pharmacokinetic/pharmacodynamic modelling-based optimisation of administration schedule for the histone deacetylase inhibitor abexinostat (S78454/PCI-24781) in phase I. Eur J Cancer 49:2791–2797
EMA Oncology Working Party. Guideline on the evaluation of anticancer medicinal products in man. European Medicines Agency 2010; www.ema.europa.eu. Accessed 30-9-2013
Fouliard S, Chenel M. BSA-adjusted dose? An old method to fight old bias. Poster presented at the Population Approach Group Europe meeting 2010. PAGE 2010; www.page-meeting.org. Accessed 30-9-2013
Evens AM, Vose JM, Harb W, Gordon LI, Langdon R, Grant B et al. A phase II multicenter study of the histone deactelase inhibitor abexinostat (PCI-24781) in relapsed/refractory follicular lymphoma (FLp and mantle cell lymphoma (MCL). Abstract 55. Blood 2013; 120 (Suppl)
Green MR, Gentles AJ, Nair RV, Irish JM, Kihira S, Liu CL et al (2013) Hierarchy in somatic mutations arising during genomic evolution and progression of follicular lymphoma. Blood 121:1604–1611
Okosun J, Bodor C, Wang J, Araf S, Yang CY, Pan C et al (2014) Integrated genomic analysis identifies recurrent mutations and evolution patterns driving the initiation and progression of follicular lymphoma. Nat Genet 46:176–181
Munster PN, Thurn KT, Thomas S, Raha P, Lacevic M, Miller A et al (2011) A phase II study of the histone deacetylase inhibitor vorinostat combined with tamoxifen for the treatment of patients with hormone therapy-resistant breast cancer. Br J Cancer 104:1828–1835
Disclosures
F.M., E.B., and A.V.: no relevant conflicts of interest to disclose. L.T.: honoraria from Servier, Amgen, and GSK; consultancy for Pfizer. B.C. consultancy, honoraria, and research funding for Celgene; honoraria from Servier. I.K., H.L., A-L.S. and S.D.: employees of Servier. V.R.: membership of board of directors or advisory committees for Servier, AstraZeneca, and Takeda; research funding from Servier, Bayer, and Sanofi.
Role of the funding source
All authors participated in the study design, the interpretation of the results, the development and writing of the manuscript, and the decision to submit for publication. The sponsor was responsible for data management and the final data analyses.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table 1S
Plasma pharmacokinetic parameters for abexinostat. Cmax, maximum concentration achieved for sampling time up to 4 hours tmax, time in hours taken to reach maximum concentration. AUC24, area under the curve for sampling time up to 24 hours t½z, terminal elimination half-life. NA: not applicable. (DOC 28 kb)
Rights and permissions
About this article
Cite this article
Morschhauser, F., Terriou, L., Coiffier, B. et al. Phase 1 study of the oral histone deacetylase inhibitor abexinostat in patients with Hodgkin lymphoma, non-Hodgkin lymphoma, or chronic lymphocytic leukaemia. Invest New Drugs 33, 423–431 (2015). https://doi.org/10.1007/s10637-015-0206-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-015-0206-x