Summary
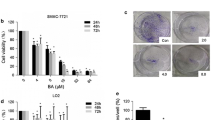
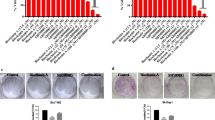
Introduction Hepatocellular carcinoma (HCC) is the most abundant tumour of the liver with rising patient numbers in the Western world countries. Despite newly approved drugs like protein kinase inhibitors the survival rate is still poor. Methods In order to identify potential new drugs for the treatment of HCC we investigated the real-time cell viability, apoptosis induction (sub-G1 cells), and HDAC (histone deacetylase) activity of two hepatocellular cancer cell lines HepG2 and Hep3B treated with new imidazole-tethered hydroxamates. Results The tested cinnamyl hydroxamates exhibited significant antiproliferative and cytotoxic activity in HCC cells as apparent from high sub-G1 cell levels in flow cytometric cell cycle analyses. In Hep3B cells HDAC inhibition was observed comparable in magnitude to that induced by the clinically applied HDAC inhibitor SAHA (Zolinza, Vorinostat). Conclusions The new imidazolyl hydroxamic acids lend themselves as a possible alternative to SAHA in the therapy of HCC. Even more so since similar 4,5-diarylimidazoles lacking only the hydroxamate functionality were previously shown in animal studies to be well tolerated and orally applicable.





Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA Cancer J Clin 62:10–29
Bosetti C, Levi F, Boffetta P, Lucchini F, Negri E, La Vecchia C (2008) Trends in mortality from hepatocellular carcinoma in Europe, 1980–2004. Hepatology 48:137–145
El-Serag HB, Rudolph KL (2007) Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 132:2557–2576
Duffy A, Greten T (2010) Developing better treatments in hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol 4:551–560
Montalbano R, Waldegger P, Quint K, Jabari S, Neureiter D, Illig R, Ocker M, Di Fazio P (2013) Endoplasmic reticulum stress plays a pivotal role in cell death mediated by the pan-deacetylase inhibitor panobinostat in human hepatocellular cancer cells. Transl Oncol 6:143–157
Di Fazio P, Schneider-Stock R, Neureiter D, Okamoto K, Wissniowski T, Gahr S, Quint K, Meissnitzer M, Alinger B, Montalbano R, Sass G, Hohenstein B, Hahn EG, Ocker M (2010) The pan-deacetylase inhibitor panobinostat inhibits growth of hepatocellular carcinoma models by alternative pathways of apoptosis. Cell Oncol 32:285–300
Zopf S, Ocker M, Neureiter D, Alinger B, Gahr S, Neurath MF, Di Fazio P (2012) Inhibition of DNA methyltransferase activity and expression by treatment with the pan-deacetylase inhibitor panobinostat in hepatocellular carcinoma cell lines. BMC Cancer 12:386
Di Fazio P, Montalbano R, Quint K, Alinger B, Kemmerling R, Kiesslich T, Ocker M, Neureiter D (2013) The pan-deacetylase inhibitor panobinostat modulates the expression of epithelial-mesenchymal transition markers in hepatocellular carcinoma models. Oncol Lett 5:127–134
Hong L, Schroth GP, Matthews HR, Yau P, Bradbury EM (1993) Studies of the DNA binding properties of histone H4 amino terminus. Thermal denaturation studies reveal that acetylation markedly reduces the binding constant of the H4 “tail” to DNA. J Biol Chem 268:305–314
Vidali G, Boffa LC, Bradbury EM, Allfrey VG (1978) Butyrate suppression of histone deacetylation leads to accumulation of multiacetylated forms of histones H3 and H4 and increased DNase I sensitivity of the associated DNA sequences. Proc Natl Acad Sci U S A 75:2239–2243
Minucci S, Pelicci PG (2006) Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments of cancer. Nat Rev Cancer 6:38–51
Glozak MA, Seto E (2007) Histone deacetylases and cancer. Oncogene 26:5420–5432
Paris M, Porcelloni M, Binaschi M, Fattori D (2008) Histone deacetylase inhibitors: from bench to clinic. J Med Chem 51:1505–1529
Mai A, Altucci L (2009) Epi-drugs to fight cancer: from chemistry to cancer treatment, the road ahead. Int J Biochem Cell Biol 41:199–213
Richon VM, Webb Y, Merger R, Sheppard T, Jursic B, Ngo L, Civoli F, Breslow R, Rifkind RA, Marks PA (1996) Second generation hybrid polar compounds are potent inducers of transformed cell differentiation. Proc Natl Acad Sci U S A 93:5705–5708
Richon VM, Emiliani S, Verdin E, Webb Y, Breslow R, Rifkind RA, Marks PA (1998) A class of hybrid polar inducers of transformed cell differentiation inhibits histone acetylases. Proc Natl Acad Sci U S A 95:3003–3007
Marks PA (2007) Discovery and development of SAHA as an anticancer agent. Oncogene 26:1351–1356
Maiso P, Carvajal-Vergara X, Ocio EM, López-Pérez R, Mateo G, Guitiérrez N, Atadja P, Pandiella A, San Miguel JF (2006) The histone deacetylase inhibitor LBH589 is a potent antimyeloma agent that overcomes drug resistance. Cancer Res 66:5781–5789
Plumb JA, Finn PW, Williams RJ, Bandara MJ, Romero MR, Watkins CJ, La Thangue NB, Brown R (2003) Pharmacodynamic response and inhibition of growth of human xenografts by the novel histone deacetylase inhibitor PXD101. Mol Cancer Ther 2:721–728
Kong D, Ahmad A, Bao B, Li Y, Banerjee S, Sarkar FH (2012) Histone deacetylase inhibitors induce epithelial-to-mesenchymal transition in prostate cancer cells. PLoS ONE 7:e45045
Schobert R, Biersack B, Dietrich A, Effenberger K, Knauer S, Mueller T (2010) 4-(3-Halo/amino-4,5-dimethoxyphenyl)-5-aryloxazoles and –N-methylimidazoles that are cytotoxic against combretastatin A resistant tumor cells and vascular disrupting in a cisplatin resistant germ cell tumor model. J Med Chem 53:6595–6602
Biersack B, Muthukumar Y, Schobert R, Sasse F (2011) Cytotoxic and antivascular 1-methyl-4-(3-fluoro-4-methoxyphenyl)-5-(halophenyl)-imidazoles. Bioorg Med Chem Lett 21:6270–6273
Conflict of interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
The online version of this article contains supplementary material (synthesis of imidazoles 1 and 2, real time cell viability of Hep3B cells), which is available to authorized users.
ESM 1
(DOC 1842 kb)
Rights and permissions
About this article
Cite this article
Di Fazio, P., Lingelbach, S., Schobert, R. et al. 4,5-Diaryl imidazoles with hydroxamic acid appendages as anti-hepatoma agents. Invest New Drugs 33, 104–108 (2015). https://doi.org/10.1007/s10637-014-0188-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-014-0188-0