Abstract
Background
Crosstalk between tumor cells and their microenvironment plays a crucial role in the progression of hepatocellular carcinoma (HCC). Hypoxia, a common feature of advanced HCC, has been shown to modulate the evolution of the tumor microenvironment. In this study, we investigated the effect of hypoxia on tumor-stroma crosstalk in HCC.
Methods
Human HCC cell lines (Huh-BAT, SNU-475) were cocultured with an activated human hepatic stellate cell line (HSCs; LX-2) under either normoxic or hypoxic conditions. Cell growth was evaluated with the MTS assay. Apoptotic signaling cascades were assessed by immunoblot analysis. Expression of CD31 and phosphorylated (p-) Akt in HCC tissues was detected by immunohistochemistry.
Results
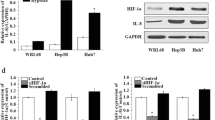
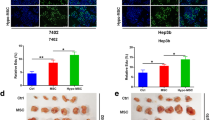
Coculturing HCC cells with HSCs under hypoxic conditions enhanced their proliferation, migration, and resistance to bile acid (BA)-induced apoptosis compared to coculturing under normoxic conditions. Under hypoxia, of various HSC-derived growth factors, PDGF-BB was the most up-regulated, leading to the activation of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway in HCC cells. Immunohistochemical study also revealed that p-Akt was highly expressed in hypoxic, hypovascular HCC as compared to hypervascular HCC. Neutralizing antisera to PDGF-BB or a PI3K inhibitor attenuated the proliferation of HCC cells cocultured with HSCs, and sensitized HCC cells to BA-induced apoptosis, especially under hypoxic conditions.
Conclusions
In conclusion, hypoxic HSC-derived PDGF-BB stimulates the proliferation of HCC cells through activation of the PI3K/Akt pathway, while the inhibition of PDGF-BB or PI3K/Akt pathways enhances apoptotic cell death. Targeting tumor-stroma crosstalk might be a novel therapy in the management of human HCCs.







Similar content being viewed by others
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- HSC:
-
Hepatic stellate cell
- MTS:
-
3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxy-methoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- DAPI:
-
4′,6-Diamidino-2-phenylindole
- BA:
-
Bile acid
- ELISA:
-
Enzyme-linked immunosorbent assay
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
- Akt:
-
Protein kinase B
- PTEN:
-
Phosphatase and tensin homolog
- PI3K:
-
Phosphoinositide 3-kinase
- PDGF:
-
Platelet-derived growth factor
- FGF:
-
Fibroblast growth factor
- TGF:
-
Transforming growth factor
- CTGF:
-
Connective tissue growth factor
- MAPK:
-
Mitogen-activated protein kinase
- OD:
-
Optical density
- SD:
-
Standard deviation
- IHC:
-
Immunohistochemical
- FOXO:
-
Forkhead transcription factors
References
El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27–S34.
Bruix J, Boix L, Sala M, Llovet JM. Focus on hepatocellular carcinoma. Cancer Cell. 2004;5:215–219.
Theret N, Musso O, Turlin B, et al. Increased extracellular matrix remodeling is associated with tumor progression in human hepatocellular carcinomas. Hepatology. 2001;34:82–88.
Eiro N, Vizoso FJ. Importance of tumor/stroma interactions in prognosis of hepatocellular carcinoma. Hepatobiliary Surg Nutr. 2014;3:98–101.
Coulouarn C, Clement B. Stellate cells and the development of liver cancer: therapeutic potential of targeting the stroma. J Hepatol. 2014;60:1306–1309.
Pinzani M, Macias-Barragan J. Update on the pathophysiology of liver fibrosis. Expert Rev Gastroenterol Hepatol. 2010;4:459–472.
Gupta DK, Singh N, Sahu DK. TGF-beta mediated crosstalk between malignant hepatocyte and tumor microenvironment in hepatocellular carcinoma. Cancer Growth Metastasis. 2014;7:1–8.
Lee HJ, Kang HJ, Kim KM, et al. Fibroblast growth factor receptor isotype expression and its association with overall survival in patients with hepatocellular carcinoma. Clin Mol Hepatol. 2015;21:60–70.
Lal A, Peters H, St Croix B, et al. Transcriptional response to hypoxia in human tumors. J Natl Cancer Inst. 2001;93:1337–1343.
Gwak GY, Yoon JH, Kim KM, Lee HS, Chung JW, Gores GJ. Hypoxia stimulates proliferation of human hepatoma cells through the induction of hexokinase II expression. J Hepatol. 2005;42:358–364.
Kim MS, Kwon HJ, Lee YM, et al. Histone deacetylases induce angiogenesis by negative regulation of tumor suppressor genes. Nat Med. 2001;7:437–443.
Stoeltzing O, Ahmad SA, Liu W, et al. Angiopoietin-1 inhibits vascular permeability, angiogenesis, and growth of hepatic colon cancer tumors. Cancer Res. 2003;63:3370–3377.
Lee JS. The mutational landscape of hepatocellular carcinoma. Clin Mol Hepatol. 2015;21:220–229.
Nakabayashi H, Taketa K, Miyano K, Yamane T, Sato J. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 1982;42:3858–3863.
Park JG, Lee JH, Kang MS, et al. Characterization of cell lines established from human hepatocellular carcinoma. Int J Cancer. 1995;62:276–282.
Xu L, Hui AY, Albanis E, et al. Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut. 2005;54:142–151.
Chung GE, Lee JH, Yoon JH, et al. Prognostic implications of tumor vascularity and its relationship to cytokeratin 19 expression in patients with hepatocellular carcinoma. Abdom Imaging. 2012;37:439–446.
Liang Y, Zheng T, Song R, et al. Hypoxia-mediated sorafenib resistance can be overcome by EF24 through Von Hippel–Lindau tumor suppressor-dependent HIF-1alpha inhibition in hepatocellular carcinoma. Hepatology. 2013;57:1847–1857.
Tezuka M, Hayashi K, Kubota K, et al. Growth rate of locally recurrent hepatocellular carcinoma after transcatheter arterial chemoembolization: comparing the growth rate of locally recurrent tumor with that of primary hepatocellular carcinoma. Dig Dis Sci. 2007;52:783–788.
Amann T, Bataille F, Spruss T, et al. Activated hepatic stellate cells promote tumorigenicity of hepatocellular carcinoma. Cancer Sci. 2009;100:646–653.
Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562.
Campbell JS, Hughes SD, Gilbertson DG, et al. Platelet-derived growth factor C induces liver fibrosis, steatosis, and hepatocellular carcinoma. Proc Natl Acad Sci USA. 2005;102:3389–3394.
Vaillancourt RR, Gardner AM, Kazlauskas A, Johnson GL. The kinase-inactive PDGF beta-receptor mediates activation of the MAP kinase cascade via the endogenous PDGF alpha-receptor in HepG2 cells. Oncogene. 1996;13:151–159.
Maass T, Thieringer FR, Mann A, et al. Liver specific overexpression of platelet-derived growth factor-B accelerates liver cancer development in chemically induced liver carcinogenesis. Int J Cancer. 2011;128:1259–1268.
Acknowledgments
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry for Health and Welfare, Republic of Korea (1420050), and by the Liver Research Foundation of Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors disclose no conflicts of interest.
Additional information
Yuri Cho and Eun Ju Cho have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Cho, Y., Cho, E.J., Lee, JH. et al. Hypoxia Enhances Tumor-Stroma Crosstalk that Drives the Progression of Hepatocellular Carcinoma. Dig Dis Sci 61, 2568–2577 (2016). https://doi.org/10.1007/s10620-016-4158-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-016-4158-6