Abstract
Background and Aims
PepT1 can transport bacterial oligopeptide products and induce intestinal inflammation. Our aim was to investigate the mechanism of the small intestine injury induced by bacterial oligopeptide product muramyl dipeptide (MDP) which is transported by PepT1.
Methods
We perfused the jejunum with a solution with or without MDP, or with a solution of MDP + Gly-Gly and explored the degree of inflammation to determine the role of PepT1–Nod2 signaling pathway in small intestine mucosa.
Results
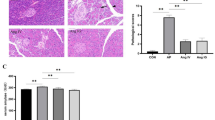
MDP perfusion induced inflammatory cell accumulation and intestinal damage, accompanied by an increase in mucosal Nod2 and Rip2 transcript expression. NFκB activity and inflammatory cytokine expression, including serum levels of TNF-α, IL-1β, and IL-6, increased in the MDP group compared to the controls; these effects were reversed by perfusion of the nutritional dipeptide Gly-Gly.
Conclusion
MDP can be transported through PepT1, causing inflammatory damage in the rat small intestine. Nod2–Rip2–NFκB signaling involved in the small intestinal inflammatory injury caused by MDP which is transported through PepT1.



Similar content being viewed by others
References
Deitch EA. Bacterial translocation or lymphatic drainage of toxic products from the gut: what is important in human beings? Surgery. 2002;131:241–244.
Gatt M, Reddy BS, MacFie J. Review article: bacterial translocation in the critically ill–evidence and methods of prevention. Aliment Pharmacol Ther. 2007;25:741–757.
Balzan S, de Almeida QuadrosC, de Cleva R, et al. Bacterial translocation: overview of mechanisms and clinical impact. J Gastroenterol Hepatol. 2007;22:464–471.
Buyse M, Tsocas A, Walker F, et al. PepT1-mediated fMLP transport induces intestinal inflammation in vivo. Am J Physiol Cell Physiol. 2002;283:C1795–1800.
Merlin D, Steel A, Gewirtz AT, et al. hPepT1-mediated epithelial transport of bacteria-derived chemotactic peptides enhances neutrophil-epithelial interactions. J Clin Invest. 2008;102:2011–2018.
Chamaillard M, Girardin SE, Viala J, et al. Nods, Nalps and Naip: intracellular regulators of bacterial-induced inflammation. Cell Microbiol. 2003;5:581–592.
Girardin SE, Boneca IG, Viala J, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–8872.
Laroui H, Yan Y, Narui Y, et al. L-Ala-γ-D-Glu-meso-diaminopimelic acid (DAP) interacts directly with leucine-rich region domain of nucleotide-binding oligomerization domain 1, increasing phosphorylation activity of receptor-interacting serine/threonine-protein kinase 2 and its interaction with nucleotide-binding oligomerization domain 1. J Biol Chem. 2011;286:31003–31013.
Vavricka SR, Musch MW, Chang JE, et al. hPepT1 transports muramyl dipeptide, activating NF-kappaB and stimulating IL-8 secretion in human colonic Caco2/bbe cells. Gastroenterology. 2004;127:1401–1409.
Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–1350.
Adibi SA. Regulation of expression of the intestinal oligopeptide transporter (Pept-1) in health and disease. Am J Physiol Gastrointest Liver Physiol. 2003;285:G779–788.
Dalmasso G, Nguyen HT, Charrier-Hisamuddin L, et al. PepT1 mediates transport of the proinflammatory bacterial tripeptide L-Ala-{gamma}-D-Glu-meso-DAP in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2010;299:G687–696.
Shi B, Song D, Xue H, Li J, Li N, Li J. Abnormal expression of the peptide transporter PepT1 in the colon of massive bowel resection rat: a potential route for colonic mucosa damage by transport of fMLP. Dig Dis Sci. 2006;51:2087–2093.
Shi B, Song D, Xue H, et al. PepT1 mediates colon damage by transporting fMLP in rats with bowel resection. J Surg Res. 2006;136:38–44.
Clark JA, Coopersmith CM. Intestinal crosstalk: a new paradigm for understanding the gut as the “motor” of critical illness. Shock. 2007;28:384–393.
Fine J, Frank ED, Ravin HA, et al. The bacterial factor in traumatic shock. N Engl J Med. 1959;260:214–220.
Merlin D, Si-Tahar M, Sitaraman SV, et al. Colonic epithelial hPepT1 expression occurs in inflammatory bowel disease: transport of bacterial peptides influences expression of MHC class 1 molecules. Gastroenterology. 2001;120:1666–1679.
Husebye E. The pathogenesis of gastrointestinal bacterial overgrowth. Chemotherapy. 2005;51:1–22.
Marshall JC, Christou NV, Horn R, et al. The microbiology of multiple organ failure. The proximal gastrointestinal tract as an occult reservoir of pathogens. Arch Surg. 1988;123:309–315.
Rosenstiel P, Till A, Schreiber S. NOD-like receptors and human diseases. Microbes Infect. 2007;9:648–657.
Dalmasso G, Nguyen HT, Ingersoll SA, et al. The PepT1-NOD2 signaling pathway aggravates induced colitis in mice. Gastroenterology. 2011;141:1334–1345.
Inohara N, Koseki T, Lin J, et al. An induced proximity model for NF-kappa B activation in the Nod1/RICK and RIP signaling pathways. J Biol Chem. 2000;275:27823–27831.
Yin X, Krikorian P, Logan T, et al. Induction of RIP-2 kinase by proinflammatory cytokines is mediated via NF-kappaB signaling pathways and involves a novel feed-forward regulatory mechanism. Mol Cell Biochem. 2010;333:251–259.
Buyse M, Berlioz F, Guilmeau S, et al. PepT1-mediated epithelial transport of dipeptides and cephalexin is enhanced by luminal leptin in the small intestine. J Clin Invest. 2001;108:1483–1494.
Franchi L, Warner N, Viani K, et al. Function of Nod-like receptors in microbial recognition and host defense. Immunol Rev. 2009;227:106–128.
Tanabe T, Chamaillard M, Ogura Y, et al. Regulatory regions and critical residues of NOD2 involved in muramyl dipeptide recognition. EMBO J. 2004;23:1587–1597.
Acknowledgments
This study was supported by Grant 12SJGGYY09 from the science research project of Songjiang District Science and Technology Committee of Shanghai (12SJGGYY09).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guoguang Ma and Bin Shi have contributed to the work equally and should be regarded as joint first authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ma, G., Shi, B., Liu, J. et al. Nod2–Rip2 Signaling Contributes to Intestinal Injury Induced by Muramyl Dipeptide Via Oligopeptide Transporter in Rats. Dig Dis Sci 60, 3264–3270 (2015). https://doi.org/10.1007/s10620-015-3762-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-015-3762-1