Abstract
Background and Aim
The incidence of gastric neuroendocrine tumors (NETs) has increased tenfold since the 1970s. Our aim was to describe the clinicopathologic profile, management, and outcomes of type I gastric NETs at The Mount Sinai Hospital.
Methods
From existing databases of the Mount Sinai Division of Gastrointestinal Pathology and the Carcinoid Cancer Foundation, we identified 56 patients with type I gastric NETs seen at The Mount Sinai Hospital from 1993 to 2012. We generated a comprehensive dataset encompassing demographic, clinical, endoscopic, and pathologic factors. Survival information was determined from medical records and the Social Security Death Index. Tumor–node–metastasis staging was conducted, and tumors were graded based on mitotic counts and Ki67 index.
Results
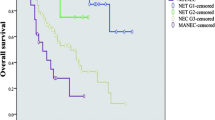
Median NET size was 3.0 mm; 55.8 % displayed multifocal disease. Stages I, II, III, and IV disease were observed in 83.8, 10.8, 5.4, and 0 %, respectively. Tumors were either low (69.7 %) or intermediate (30.3 %) grade. Furthermore, 3.6 % of patients developed gastric dysplasia, and 5.5 % had gastric adenocarcinoma. Patients underwent endoscopy every 15 months, while 28.6 % underwent polypectomy, 32.7 % somatostatin therapy, and 46.4 % surgical resection. 5- and 10-year disease-specific survival was 100 %.
Conclusions
Most patients received annual endoscopic surveillance, with a minority undergoing surgical resection, though outcomes remained excellent independent of therapeutic approach. We identified a very low but real rate of loco-regional spread, despite the generally indolent behavior of type I gastric NETs. Several patients demonstrated concurrent dysplasia or adenocarcinoma, underscoring the efficacy of regular endoscopic management not only for gastric NETs, but also for dysplasia and adenocarcinoma.


Similar content being viewed by others
Abbreviations
- AJCC:
-
American Joint Committee on Cancer
- Anti-IF:
-
Intrinsic factor antibody
- Anti-PC:
-
Parietal cell antibody
- ECL:
-
Enterochromaffin-like
- EGD:
-
Esophagogastroduodenoscopy
- ENETS:
-
European Neuroendocrine Tumor Society
- EUS:
-
Endoscopic ultrasound
- GEP-NET:
-
Gastroenteropancreatic neuroendocrine tumor
- MALT:
-
Mucosa-associated lymphoid tissue
- NET:
-
Neuroendocrine tumor
- PPI:
-
Proton-pump inhibitor
- TNM:
-
Tumor–node–metastasis
References
Merola E, Sbrozzi-Vanni A, Panzuto F, et al. Type I gastric carcinoids: a prospective study on endoscopic management and recurrence rate. Neuroendocrinology. 2012;95:207–213.
Ozao-Choy J, Buch K, Strauchen JA, et al. Laparoscopic antrectomy for the treatment of type I gastric carcinoid tumors. J Surg Res. 2010;162:22–25.
Rindi G, Azzoni C, La Rosa S, et al. ECL cell tumor and poorly differentiated endocrine carcinoma of the stomach: prognostic evaluation by pathological analysis. Gastroenterology. 1999;116:532–542.
Dakin GF, Warner RR, Pomp A, et al. Presentation, treatment, and outcome of type 1 gastric carcinoid tumors. J Surg Oncol. 2006;93:368–372.
Gladdy RA, Strong VE, Coit D, et al. Defining surgical indications for type I gastric carcinoid tumor. Ann Surg Oncol. 2009;16:3154–3160.
Saund MS, Al Natour RH, Sharma AM, et al. Tumor size and depth predict rate of lymph node metastasis and utilization of lymph node sampling in surgically managed gastric carcinoids. Ann Surg Oncol. 2011;18:2826–2832.
Solcia E, Rindi G, Silini E, et al. Enterochromaffin-like (ECL) cells and their growths: relationships to gastrin, reduced acid secretion and gastritis. Baillieres Clin Gastroenterol. 1993;7:149–165.
Rindi G, Luinetti O, Cornaggia M, et al. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology. 1993;104:994–1006.
Modlin IM, Lye KD, Kidd M. A 50-year analysis of 562 gastric carcinoids: small tumor or larger problem? Am J Gastroenterol. 2004;99:23–32.
Lawrence B, Gustafsson BI, Chan A, et al. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:1–18.
Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959.
Modlin IM, Sandor A. An analysis of 8305 cases of carcinoid tumors. Cancer. 1997;79:813–829.
Modlin IM, Sandor A, Tang LH, et al. A 40-year analysis of 265 gastric carcinoids. Am J Gastroenterol. 1997;92:633–638.
Hodgson N, Koniaris LG, Livingstone AS, et al. Gastric carcinoids: a temporal increase with proton pump introduction. Surg Endosc. 2005;19:1610–1612.
Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072.
Maggard MA, O’Connell JB, Ko CY. Updated population-based review of carcinoid tumors. Ann Surg. 2004;240:117–122.
Sjoblom SM, Sipponen P, Jarvinen H. Gastroscopic follow up of pernicious anaemia patients. Gut. 1993;34:28–32.
Borch K, Ahren B, Ahlman H, et al. Gastric carcinoids: biologic behavior and prognosis after differentiated treatment in relation to type. Ann Surg. 2005;242:64–73.
Hirschowitz BI, Griffith J, Pellegrin D, et al. Rapid regression of enterochromaffin like cell gastric carcinoids in pernicious anemia after antrectomy. Gastroenterology. 1992;102:1409–1418.
Higham AD, Dimaline R, Varro A, et al. Octreotide suppression test predicts beneficial outcome from antrectomy in a patient with gastric carcinoid tumor. Gastroenterology. 1998;114:817–822.
Ferraro G, Annibale B, Marignani M, et al. Effectiveness of octreotide in controlling fasting hypergastrinemia and related enterochromaffin-like cell growth. J Clin Endocrinol Metab. 1996;81:677–683.
Granberg D, Wilander E, Stridsberg M, et al. Clinical symptoms, hormone profiles, treatment, and prognosis in patients with gastric carcinoids. Gut. 1998;43:223–228.
Soga J. Early-stage carcinoids of the gastrointestinal tract: an analysis of 1914 reported cases. Cancer. 2005;103:1587–1595.
Jordan PH Jr, Barroso A, Sweeney J. Gastric carcinoids in patients with hypergastrinemia. J Am Coll Surg. 2004;199:552–555.
Kulke MH, Anthony LB, Bushnell DL, et al. NANETS treatment guidelines: well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas. 2010;39:735–752.
Klimstra DS, Modlin IR, Coppola D, et al. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707–712.
Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer; 2010.
Carmel R. Reassessment of the relative prevalences of antibodies to gastric parietal cell and to intrinsic factor in patients with pernicious anaemia: influence of patient age and race. Clin Exp Immunol. 1992;89:74–77.
Ottesen M, Feldt-Rasmussen UF, Andersen J, et al. Pernicious anemia. A study of initial forms of the disease and diagnostic significance of determination of the intrinsic factor antibody and parietal cell antibody. Ugeskr Laeger. 1992;154:3758–3762.
Schindl M, Kaserer K, Niederle B. Treatment of gastric neuroendocrine tumors: the necessity of a type-adapted treatment. Arch Surg. 2001;136:49–54.
Wiedenmann B, Jensen RT, Mignon M, et al. Preoperative diagnosis and surgical management of neuroendocrine gastroenteropancreatic tumors: general recommendations by a consensus workshop. World J Surg. 1998;22:309–318.
Zissis D, Zizi-Serbetzoglou A, Grammatoglou X, et al. Combined carcinoid and mixed (composite) glandular-endocrine cell carcinoma of the stomach in atrophic gastritis. J BUON. 2009;14:127–130.
Ronellenfitsch U, Strobel P, Schwarzbach MH, et al. A composite adenoendocrine carcinoma of the stomach arising from a neuroendocrine tumor. J Gastrointest Surg. 2007;11:1573–1575.
Jain D, Eslami-Varzaneh F, Takano AM, et al. Composite glandular and endocrine tumors of the stomach with pancreatic acinar differentiation. Am J Surg Pathol. 2005;29:1524–1529.
Yang GC, Rotterdam H. Mixed (composite) glandular-endocrine cell carcinoma of the stomach. Report of a case and review of literature. Am J Surg Pathol. 1991;15:592–598.
Lee EJ, Park SM, Maeng L, et al. Composite glandular-endocrine cell carcinomas of the stomach: clinicopathologic and methylation study. APMIS. 2005;113:569–576.
de Vries AC, van Grieken NC, Looman CW, et al. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology. 2008;134:945–952.
Correa P. Human gastric carcinogenesis: a multistep and multifactorial process—First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–6740.
Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789.
Leung WK, Sung JJ. Review article: intestinal metaplasia and gastric carcinogenesis. Aliment Pharmacol Ther. 2002;16:1209–1216.
Kulke MH, Benson AB 3rd, Bergsland E, et al. Neuroendocrine tumors. J Natl Compr Canc Netw. 2012;10:724–764.
Hsing AW, Hansson LE, McLaughlin JK, et al. Pernicious anemia and subsequent cancer. A population-based cohort study. Cancer. 1993;71:745–750.
Waldum HL, Aase S, Kyetnoi I, et al. Neuroendocrine differentiation in human gastric carcinoma. Cancer. 1998;83:435–444.
Modlin IM, Lye KD, Kidd M. Carcinoid tumors of the stomach. Surg Oncol. 2003;12:153–172.
Acknowledgments
We are grateful to National Center for Advancing Translational Sciences (NCATS) KL2TR000069.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, W.C., Warner, R.R.P., Ward, S.C. et al. Management and Disease Outcome of Type I Gastric Neuroendocrine Tumors: The Mount Sinai Experience. Dig Dis Sci 60, 996–1003 (2015). https://doi.org/10.1007/s10620-014-3410-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-014-3410-1